Incidence and Trends of Infection with Pathogens Transmitted Commonly Through Food — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2006–2013
Stacy M. Crim, MPH1, Martha Iwamoto, MD1, Jennifer Y. Huang, MPH1, Patricia M. Griffin, MD1, Debra Gilliss, MD2, Alicia B. Cronquist, MPH3, Matthew Cartter, MD4, Melissa Tobin-D'Angelo, MD5, David Blythe, MD6, Kirk Smith, DVM7, Sarah Lathrop, PhD8, Shelley Zansky, PhD9, Paul R. Cieslak, MD10, John Dunn, DVM11, Kristin G. Holt, DVM12, Susan Lance, DVM13, Robert Tauxe, MD1, Olga L. Henao, PhD1 (Author affiliations at end of text)
Foodborne disease continues to be an important problem in the United States. Most illnesses are preventable. To evaluate progress toward prevention, the Foodborne Diseases Active Surveillance Network* (FoodNet) monitors the incidence of laboratory-confirmed infections caused by nine pathogens transmitted commonly through food in 10 U.S. sites, covering approximately 15% of the U.S. population. This report summarizes preliminary 2013 data and describes trends since 2006. In 2013, a total of 19,056 infections, 4,200 hospitalizations, and 80 deaths were reported. For most infections, incidence was well above national Healthy People 2020 incidence targets and highest among children aged <5 years. Compared with 2010–2012, the estimated incidence of infection in 2013 was lower for Salmonella, higher for Vibrio, and unchanged overall.† Since 2006–2008, the overall incidence has not changed significantly. More needs to be done. Reducing these infections requires actions targeted to sources and pathogens, such as continued use of Salmonella poultry performance standards and actions mandated by the Food Safety Modernization Act (FSMA) (1). FoodNet provides federal and state public health and regulatory agencies as well as the food industry with important information needed to determine if regulations, guidelines, and safety practices applied across the farm-to-table continuum are working.
FoodNet conducts active, population-based surveillance for laboratory-confirmed infections caused by Campylobacter, Cryptosporidium, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC) O157 and non-O157, Shigella, Vibrio, and Yersinia in 10 sites covering approximately 15% of the U.S. population (an estimated 48 million persons in 2012).§ FoodNet is a collaboration among CDC, 10 state health departments, the U.S. Department of Agriculture's Food Safety and Inspection Service (USDA-FSIS), and the Food and Drug Administration (FDA). Hospitalizations occurring within 7 days of specimen collection are recorded, as is the patient's vital status at hospital discharge, or at 7 days after specimen collection if the patient was not hospitalized. Hospitalizations and deaths that occur within 7 days are attributed to the infection. Surveillance for physician-diagnosed postdiarrheal hemolytic uremic syndrome (HUS), a complication of STEC infection characterized by renal failure, is conducted through a network of nephrologists and infection preventionists and by hospital discharge data review. This report includes 2012 HUS data for persons aged <18 years.
Incidence was calculated by dividing the number of laboratory-confirmed infections in 2013 by U.S. Census estimates of the surveillance area population for 2012.¶ Incidence of culture-confirmed bacterial infections and laboratory-confirmed parasitic infections (e.g., identified by enzyme immunoassay) are reported. A negative binomial model with 95% confidence intervals (CIs) was used to estimate changes in incidence from 2010–2012 to 2013 and from 2006–2008 to 2013 (2). Change in the overall incidence of infection with six key foodborne pathogens was estimated (3). For STEC non-O157, only change since 2010–2012 was assessed because diagnostic practices changed before then; for Cyclospora, change was not assessed because data were sparse. For HUS, incidence was compared with 2006–2008. The number of reports of positive culture-independent diagnostic tests (CIDTs) without corresponding culture confirmation is included for Campylobacter, Listeria, Salmonella, Shigella, STEC, Vibrio, and Yersinia.
Cases of Infection, Incidence, and Trends
In 2013, FoodNet identified 19,056 cases of infection, 4,200 hospitalizations, and 80 deaths (Table). The number and incidence per 100,000 population were Salmonella (7,277 [15.19]), Campylobacter (6,621 [13.82]), Shigella (2,309 [4.82]), Cryptosporidium (1,186 [2.48]), STEC non-O157 (561 [1.17]), STEC O157 (552 [1.15]), Vibrio (242 [0.51]), Yersinia (171 [0.36]), Listeria (123 [0.26]), and Cyclospora (14 [0.03]). Incidence was highest among persons aged ≥65 years for Cyclospora, Listeria, and Vibrio and among children aged <5 years for all the other pathogens.
Among 6,520 (90%) serotyped Salmonella isolates, the top serotypes were Enteritidis, 1,237 (19%); Typhimurium, 917 (14%); and Newport, 674 (10%). Among 231 (95%) speciated Vibrio isolates, 144 (62%) were V. parahaemolyticus, 27 (12%) were V. alginolyticus, and 21 (9%) were V. vulnificus. Among 458 (82%) serogrouped STEC non-O157 isolates, the top serogroups were O26 (34%), O103 (25%), and O111 (14%).
Compared with 2010–2012, the 2013 incidence was significantly lower for Salmonella (9% decrease; CI = 3%–15%), higher for Vibrio (32% increase; CI = 8%–61%) and not significantly changed for other pathogens (Figure 1). Compared with 2006–2008, the 2013 incidence was significantly higher for Campylobacter and Vibrio (Figure 2). The overall incidence of infection with six key foodborne pathogens was not significantly different in 2013 compared with 2010–2012 or 2006–2008.
Compared with 2010–2012, the 2013 incidence of infection with specific Salmonella serotypes was significantly lower for Enteritidis (14% decrease; CI = 0.2%–25%) and Newport (32% decrease; CI = 17%–44%) and not significantly changed for Typhimurium. Compared with 2006–2008, however, the 2013 incidence of infection was significantly changed only for Typhimurium (20% decrease; CI = 10%–28%).
Among 62 cases of postdiarrheal HUS in children aged <18 years (0.56 cases per 100,000) in 2012, 38 (61%) occurred in children aged <5 years (1.27 cases per 100,000). Compared with 2006–2008, the incidence was significantly lower for children aged <5 years (36% decrease; CI = 9%–55%) and for children aged <18 years (31% decrease; CI = 7%–49%).
In addition to culture-confirmed infections (some with a positive CIDT result), there were 1,487 reports of positive CIDTs that were not confirmed by culture, either because the specimen was not cultured at either the clinical or public health laboratory or because a culture did not yield the pathogen. For 1,017 Campylobacter reports in this category, 430 (42%) had no culture, and 587 (58%) were culture-negative. For 247 STEC reports, 59 (24%) had no culture, and 188 (76%) were culture-negative. The Shiga toxin–positive result was confirmed for 65 (34%) of 192 broths sent to a public health laboratory. The other reports of positive CIDT tests not confirmed by culture were of Shigella (147), Salmonella (69), Vibrio (four), Listeria (two), and Yersinia (one).
Discussion
The incidence of laboratory-confirmed Salmonella infections was lower in 2013 than 2010–2012, whereas the incidence of Vibrio infections increased. No changes were observed for infection with Campylobacter, Listeria, STEC O157, or Yersinia, the other pathogens transmitted commonly through food for which Healthy People 2020 targets exist. The lack of recent progress toward these targets points to gaps in the current food safety system and the need for more food safety interventions.
Although the incidence of Salmonella infection in 2013 was lower than during 2010–2012, it was similar to 2006–2008, well above the national Healthy People target. Salmonella organisms live in the intestines of many animals and can be transmitted to humans through contaminated food or water or through direct contact with animals or their environments; different serotypes can have different reservoirs and sources. Enteritidis, the most commonly isolated serotype, is often associated with eggs and poultry. The incidence of Enteritidis infection was lower in 2013 compared with 2010–2012, but not compared with 2006–2008. This might be partly explained by the large Enteritidis outbreak linked to eggs in 2010.** Ongoing efforts to reduce contamination of eggs include FDA's Egg Safety Rule, which requires shell egg producers to implement controls to prevent contamination of eggs on the farm and during storage and transportation.†† FDA required compliance by all egg producers with ≥50,000 laying hens by 2010 and by producers with ≥3,000 hens by 2012. Reduction in Enteritidis infection has been one of five high-priority goals for the U.S. Department of Health and Human Services since 2012.§§
In 2013, the incidence of Vibrio infections was the highest observed in FoodNet to date, though still much lower than that of Salmonella or Campylobacter. Vibrio infections are most common during warmer months, when waters contain more Vibrio organisms. Many infections follow contact with seawater (4), but about 50% of domestically acquired infections are transmitted through food, most commonly oysters (5). Foodborne infections can be prevented by postharvest treatment of oysters with heat, freezing, or high pressure, by thorough cooking, or by not eating oysters during warmer months (6). During the summers of 2012 and 2013, many V. parahaemolyticus infections of a strain previously traced only to the Pacific Northwest were associated with consumption of oysters and other shellfish from several Atlantic Coast harvest areas.¶¶ V. alginolyticus, the second most common Vibrio reported to FoodNet in 2013, typically causes wound and soft-tissue infections among persons who have contact with water (7).
The continued decrease in the incidence of postdiarrheal HUS has not been matched by a decline in STEC O157 infections. Possible explanations include unrecognized changes in surveillance, improvements in management of STEC O157 diarrhea, or an actual decrease in infections with the most virulent strains of STEC O157. It is possible that more stool specimens are being tested for STEC, resulting in increased detection of milder infections than in the past. Continued surveillance is needed to determine if this pattern holds.
CIDTs are increasingly used by clinical laboratories to diagnose bacterial enteric infections, a trend that will challenge the ability to identify cases, monitor trends, detect outbreaks, and characterize pathogens (8). Therefore, FoodNet began tracking CIDT-positive reports and surveying clinical laboratories about their diagnostic practices. The adoption of CIDTs has varied by pathogen and has been highest for STEC and Campylobacter. Positive CIDTs frequently cannot be confirmed by culture, and the positive predictive value varies by the CIDT used. For STEC, most specimens identified as Shiga toxin–positive were sent to a public health laboratory for confirmation. However, for other pathogens the fraction of specimens from patients with a positive CIDT sent for confirmation likely is low because no national guidelines regarding confirmation of CIDT results currently exist. As the number of approved CIDTs increases, their use likely will increase rapidly. Clinicians, clinical and public health laboratorians, public health practitioners, regulatory agencies, and industry must work together to maintain strong surveillance to detect dispersed outbreaks, measure the impact of prevention measures, and identify emerging threats.
The findings in this report are subject to at least five limitations. First, health-care–seeking behaviors and other characteristics of the population in the surveillance area might affect the generalizability of the findings. Second, some agents transmitted commonly through food (e.g., norovirus) are not monitored by FoodNet because clinical laboratories do not routinely test for them. Third, the proportion of illnesses transmitted by nonfood routes differs by pathogen; data provided in this report are not limited to infections from food. Fourth, in some fatal cases, infection with the enteric pathogen might not have been the primary cause of death. Finally, changes in incidence between periods can reflect year-to-year variation during those periods rather than sustained trends.
Most foodborne illnesses can be prevented, and progress has been made in decreasing contamination of some foods and reducing illness caused by some pathogens since 1996, when FoodNet began. More can be done; surveillance data provide information on where to target prevention efforts. In 2011, USDA-FSIS tightened its performance standard for Salmonella contamination of whole broiler chickens; in 2013, 3.9% of samples tested positive (Christopher Aston, USDA-FSIS, Office of Data Integration and Food Protection; personal communication; 2014). Because most chicken is purchased as cut-up parts, USDA-FSIS conducted a nationwide survey of raw chicken parts in 2012 and calculated an estimated 24% prevalence of Salmonella (9). In 2013, USDA-FSIS released its Salmonella Action Plan that indicates that USDA-FSIS will conduct a risk assessment and develop performance standards for poultry parts during 2014, among other key activities (10). The Food Safety Modernization Act of 2011 gives FDA additional authority to regulate food facilities, establish standards for safe produce, recall contaminated foods, and oversee imported foods; it also calls on CDC to strengthen surveillance and outbreak response (1). For consumers, advice on safely buying, preparing, and storing foods prone to contamination is available online.
1Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 2California Department of Public Health; 3Colorado Department of Public Health and Environment; 4Connecticut Department of Public Health; 5Georgia Department of Public Health; 6Maryland Department of Health and Mental Hygiene; 7Minnesota Department of Health; 8University of New Mexico; 9New York State Department of Health; 10Oregon Health Authority; 11Tennessee Department of Health; 12Food Safety and Inspection Service, US Department of Agriculture; 13Center for Food Safety and Applied Nutrition, Food and Drug Administration (Corresponding author: Olga L. Henao, ohenao@cdc.gov, 404-639-3393)
Acknowledgments
Workgroup members, Foodborne Diseases Active Surveillance Network (FoodNet), Emerging Infections Program. Communications team, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Diseases; Enteric Diseases Laboratory Branch, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Diseases, CDC.
References
- Food and Drug Administration. FDA Food Safety Modernization Act. Washington, DC: US Department of Health and Human Services, Food and Drug Administration; 2011. Available at http://www.fda.gov/food/guidanceregulation/fsma/ucm247548.htm.
- Henao OL, Scallan E, Mahon B, Hoekstra RM. Methods for monitoring trends in the incidence of foodborne diseases: Foodborne Diseases Active Surveillance Network 1996–2008. Foodborne Pathog Dis 2010;7:1421–6.
- Henao OL, Crim SM, Hoekstra RM. Calculating a measure of overall change in the incidence of selected laboratory-confirmed infections with pathogens transmitted commonly through food, Foodborne Diseases Active Surveillance Network (FoodNet), 1996–2010. Clin Infect Dis 2012;54(Suppl 5):S418–20.
- Shapiro RL, Altekruse S, Hutwagner L, et al. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988–1996. J Infect Dis 1998;178:752–9.
- CDC. National enteric disease surveillance: COVIS annual summary, 2011. Atlanta, Georgia: US Department of Health and Human Services, CDC; 2013. Available at http://www.cdc.gov/ncezid/dfwed/pdfs/covis-annual-report-2011-508c.pdf.
- Vugia DJ, Tabnak F, Newton AE, et al. Impact of 2003 state regulation on raw oyster-associated Vibrio vulnificus illnesses and deaths, California, USA. Emerg Infect Dis 2013;19:1276–80.
- Dechet AM, Yu PA, Koram N, Painter J. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997–2006. Clin Infect Dis 2008;46:970–6.
- Cronquist AB, Mody RK, Atkinson R, et al. Impacts of culture-independent diagnostic practices on public health surveillance for bacterial enteric pathogens. Clin Infect Dis 2012;54(S5):S432–9.
- US Department of Agriculture, Food Safety and Inspection Service. The Nationwide Microbiological Baseline Data Collection Program: Raw Chicken Parts Survey, January 2012–August 2012. Washington, DC: US Department of Agriculture, Food Safety and Inspection Service; 2013. Available at http://www.fsis.usda.gov/wps/wcm/connect/a9837fc8-0109-4041-bd0c-729924a79201/baseline_data_raw_chicken_parts.pdf?mod=ajperes.
- US Department of Agriculture, Food Safety and Inspection Service. Strategic Performance Working Group Salmonella action plan. Washington, DC: US Department of Agriculture, Food Safety and Inspection Service; 2013. Available at http://www.fsis.usda.gov/wps/wcm/connect/aae911af-f918-4fe1-bc42-7b957b2e942a/sap-120413.pdf?mod=ajperes.
* Additional information available at http://www.cdc.gov/foodnet.
† The overall incidence of infection combines data for Campylobacter, Listeria, Salmonella, STEC O157, Vibrio, and Yersinia, six key bacterial pathogens for which >50% of illnesses are estimated to be transmitted by food.
§ FoodNet personnel regularly contact clinical laboratories to ascertain all laboratory-confirmed infections in residents of the surveillance areas.
¶ Final incidence rates will be reported when population estimates for 2013 are available.
** Additional information available at http://www.cdc.gov/salmonella/enteritidis/index.html.
†† Additional information available at http://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/eggs/ucm170615.htm.
§§ Additional information available at http://www.hhs.gov/strategic-plan/appendixb3.html.
¶¶ Additional information available at http://www.cdc.gov/vibrio/investigations/index.html.
What is already known on this topic?
The incidences of infection caused by Campylobacter, Salmonella, Shiga toxin–producing Escherichia coli O157, and Vibrio are well above their respective Healthy People 2020 targets. Foodborne illness continues to be an important public health problem.
What is added by this report?
In 2013, a total of 19,056 infections, 4,200 hospitalizations, and 80 deaths were reported to the Foodborne Diseases Active Surveillance Network (FoodNet). For most infections, incidence was highest among children aged <5 years. In 2013, compared with 2010–2012, the estimated incidence of infection was unchanged overall, lower for Salmonella, and higher for Vibrio infections, which have been increasing in frequency for many years. The number of patients being diagnosed by culture-independent diagnostic tests (CIDT) is increasing.
What are the implications for public health practice?
Reducing the incidence of foodborne infections requires greater commitment and more action to implement measures to reduce contamination of food. Monitoring the incidence of these infections is becoming more difficult because some laboratories are now using CIDTs, and some do not follow up a positive CIDT result with a culture.
FIGURE 1. Estimated percentage change in incidence of culture-confirmed bacterial and laboratory-confirmed parasitic infections in 2013 compared with average annual incidence during 2010–2012, by pathogen — Foodborne Diseases Active Surveillance Network, United States
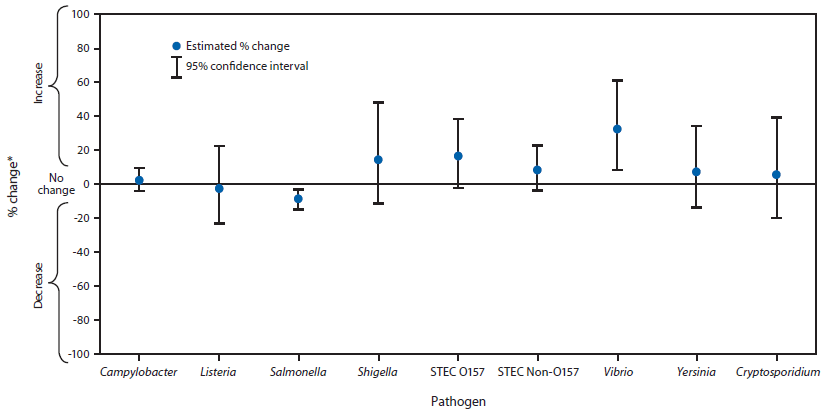
Abbreviations: CI = confidence interval; STEC = Shiga toxin–producing Escherichia coli.
* No significant change = 95% CI is both above and below the no change line; significant increase = estimate and entire CI are above the no change line; significant decrease = estimate and entire CI are below the no change line.
Alternate Text: The figure above shows estimated percentage change in incidence of culture-confirmed bacterial and laboratory-confirmed parasitic infections in 2013 compared with average annual incidence during 2010–2012, by pathogen, in the United States. Compared with 2010–2012, the 2013 incidence was significantly lower for Salmonella (9% decrease; 95% confidence interval = 3%–15%), higher for Vibrio (32% increase; 95% confidence interval = 8%–61%) and not sig¬nificantly changed for other pathogens.
FIGURE 2. Relative rates of culture-confirmed infections with Campylobacter, STEC* O157, Listeria, Salmonella, and Vibrio compared with 2006–2008 rates, by year — Foodborne Diseases Active Surveillance Network, United States, 2006–2013†
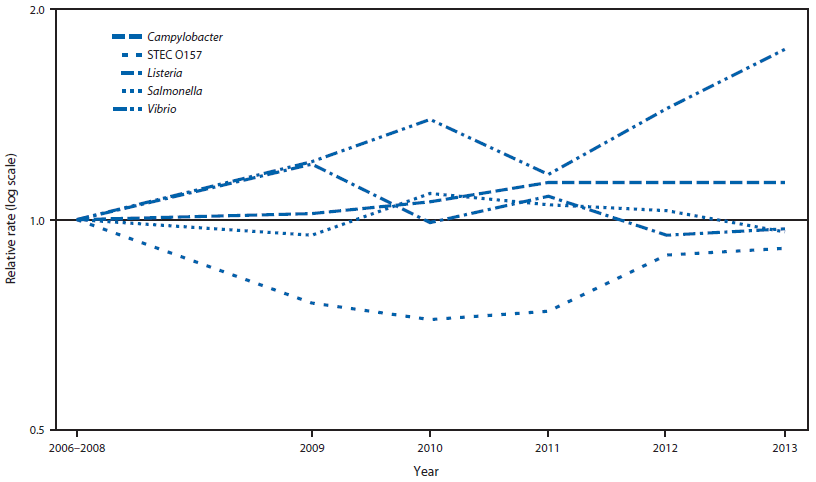
* Shiga toxin–producing Escherichia coli.
† The position of each line indicates the relative change in the incidence of that pathogen compared with 2006–2008. The actual incidences of these infections cannot be determined from this figure.
Alternate Text: The figure above shows relative rates of culture-confirmed infections with Campylobacter, Shiga toxin–producing Escherichia coli O157, Listeria, Salmonella, and Vibrio compared with 2006–2008 rates, by year, in the United States during 2006–2013. Compared with 2006–2008, the 2013 incidence was significantly higher for Campylobacter and Vibrio.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


