 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Human Tuberculosis Caused by Mycobacterium bovis --- New York City, 2001--2004In March 2004, a U.S.-born boy aged 15 months in New York City (NYC) died of peritoneal tuberculosis (TB) caused by Mycobacterium bovis infection. M. bovis, a bacterial species of the M. tuberculosis complex, is a pathogen that primarily infects cattle. However, humans also can become infected, most commonly through consumption of unpasteurized milk products from infected cows. In industrialized nations, human TB caused by M. bovis is rare because of milk pasteurization and culling of infected cattle herds (1). This report summarizes an ongoing, multiagency* investigation that has identified 35 cases of human M. bovis infection in NYC. Preliminary findings indicate that fresh cheese (e.g., queso fresco) brought to NYC from Mexico was a likely source of infection. No evidence of human-to-human transmission has been found. Products from unpasteurized cow's milk have been associated with certain infectious diseases and carry the risk of transmitting M. bovis if imported from countries where the bacterium is common in cattle. All persons should avoid consuming products from unpasteurized cow's milk†. TB SurveillanceSince January 1, 2001, spoligotyping of M. tuberculosis--complex isolates from patients with newly diagnosed TB has been conducted routinely in NYC. This rapid genotyping method is primarily used for epidemiologic monitoring; however, spoligotyping also differentiates M. bovis from M. tuberculosis. Of 4,524 TB cases reported in NYC during 2001--2004, a total of 3,417 (76%) were culture-confirmed; 3,123 (91%) of these had spoligotype results, of which 35 (1%) were M. bovis. Twelve (34%) of the M. bovis cases were in children aged <15 years (median age: 5 years), and five of the 35 cases (14%) were in children aged <5 years (range: 1--4 years). Of the 35 patients, 20 (57%) were born in Mexico, 11 (31%) in the United States, two (6%) in the Dominican Republic, and one (3%) each in Guatemala and Guyana. Of 23 adult patients (median age: 27 years; range: 16--76 years), 22 (96%) were born abroad; of the 12 patients aged <15 years, 10 (83%) were born in the United States, all of Mexican-born parents. Of the five patients aged <5 years, all had extrapulmonary disease (i.e., three lymphatic and two peritoneal). All five were born in the United States of Mexican-born parents. None had traveled outside of the United States, and no epidemiologic link to other TB cases was discovered. Twenty-six of the 35 patients received inpatient hospital care. The anatomical site of disease was extrapulmonary in 21 (60%) patients, pulmonary in nine (26%), and both pulmonary and extrapulmonary in five (14%) patients. The sputum-smear microscopy results were positive for acid-fast bacilli, indicating potential contagiousness, for eight (57%) of the 14 patients with pulmonary disease. Twenty-five (seven children and 18 adults) of the 35 patients were tested for antibodies to human immunodeficiency virus (HIV). Seven (28%) of those tested had positive HIV results; all were adults, aged 23--51 years (median: 35 years). The only fatal M. bovis case was in the boy aged 15 months. He was treated for diarrhea and fever and received inpatient and outpatient care for 4 weeks, until abdominal distension and tenderness led to laparotomy for presumed ruptured appendicitis. Tuberculous peritonitis was diagnosed on the basis of surgical and microbiologic findings, and treatment for TB was begun. However, the boy died after 4 days of treatment. During 1995--2004, the number of TB cases reported annually in NYC among Mexican-born persons ranged from 28 to 64. During 2001--2004, a total of 20 (13%) of 155 culture-confirmed TB cases in Mexican-born patients were caused by M. bovis infection, compared with 15 (<1%) of 2,925 TB cases (with spoligotype results) in all others. During 2001--2004, a total of 101 TB cases in children aged <5 years were reported; 32 (32%) of the cases were culture-confirmed, and five (16%) of the 32 culture isolates were M. bovis. The standard four-drug regimen for TB consists of isoniazid, rifampin, pyrazinamide, and ethambutol. Since 2003, a fifth drug, streptomycin, is no longer recommended as a first-line alternative to ethambutol (2). Whereas isolates of other species belonging to the M. tuberculosis complex usually are susceptible to pyrazinamide, M. bovis isolates typically are resistant. In this investigation, of the 35 isolates, 17 (49%) were resistant to pyrazinamide only; 14 (40%) were resistant to pyrazinamide and streptomycin; two (6%) were resistant to pyrazinamide, isoniazid, and streptomycin; one (3%) was resistant to pyrazinamide and isoniazid; and one (3%) had no resistance. Laboratory InvestigationIdentification of the 35 M. bovis isolates was confirmed by genetic deletion analysis. Genotyping determined nine different patterns by spoligotype, three patterns (1--7 bands) by IS6110-based restriction fragment length polymorphism (RFLP), and six patterns by mycobacterial interspersed repetitive units (MIRU). A cluster of 13 cases had identical RFLP (BE4), spoligotype (octal designation 264073777777600) (3), and MIRU (232224253322) (Figure). Genotyping with polymorphic guanine- and cytosine-rich repeat sequences (PGRS) did not reveal additional clusters. The interpretation of M. bovis genotypes for investigating paths of transmission has not been determined. Epidemiologic InvestigationOf the 35 patients, 23 (66%) patients (or parents of patients) were interviewed regarding exposures associated with M. bovis infection. Among the 12 not interviewed, two had died, three had moved back to Mexico, five had their telephones disconnected and attempts to visit them at home were unsuccessful, and two lacked usable locating information. Parents of the 10 U.S.-born children and one of the two children born abroad were interviewed, as were 12 of 22 adults. No linkages that might allow airborne, person-to-person transmission of M. bovis were discovered among any of the patients. Nineteen (83%) of the 23 interviewed reported eating cheeses produced in Mexico while they were living in the United States, including parents of four (80%) of the five children aged <5 years. The cheeses were believed obtained from one or more of the following sources: a courier agency delivering Mexican products, a visitor carrying food in luggage, a Mexican-specialty grocery, or a door-to-door vendor in NYC. Eighteen (78%) of the 23 interviewed did not know whether milk products they consumed were pasteurized. Samples of cheeses produced in Mexico and acquired in NYC are being tested for presence of M. bovis. Reported by: A Winters, MD, C Driver, DrPH, M Macaraig, MPH, C Clark, MPH, SS Munsiff, MD, C Pichardo, Bur of TB Control, New York City Dept of Health and Mental Hygiene; J Driscoll, PhD, M Salfinger, MD, Wadsworth Center, New York State Dept of Health. B Kreiswirth, PhD, Public Health Research Institute, Newark, New Jersey. J Jereb, MD, P LoBue, MD, Div of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention; M Lynch, MD, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, CDC. Editorial Note:M. bovis transmission from cattle to humans was once common in the United States, but human infections were virtually eliminated by decades of disease control in cattle herds and by routine pasteurization of cow's milk (1). Now the majority of persons who have M. bovis TB come from countries where the infection is prevalent in cattle and where they presumably acquired infection. However, in San Diego, California, during 1980--1997, 34% of culture-confirmed TB cases in children aged <15 years were caused by M. bovis; approximately 90% of these children were U.S. born and of Hispanic ethnicity (4). Fresh cheese brought from Mexico is suspected to be one source of infections in these children. The investigation in NYC, where the Mexican population tripled to 186,872 during 1990--2000, suggests that fresh cheese from Mexico might account for a high percentage of the 35 cases described in this report; however, further epidemiologic investigations and laboratory results are needed for confirmation. M. bovis causes disease in cattle, deer, and other mammals. In humans, consumption of unpasteurized infected cow's milk products can cause infection. Although human disease caused by M. bovis and other species of M. tuberculosis complex are similar, the anatomic site of M. bovis disease is more often extrapulmonary. Epidemiologic evidence supports the likelihood of human-to-human, airborne M. bovis transmission from patients who have pulmonary disease, but its relative contribution to new infections in humans is unknown (5). The frequency of isoniazid resistance in the cases described in this report was comparable to that previously reported for M. bovis in San Diego. Streptomycin resistance, which had not been examined previously for M. bovis in the United States, was approximately six times more frequent among the cases in NYC (16 of 35 isolates) than that reported for M. tuberculosis complex previously (6). Continued surveillance for drug resistance is needed to ensure effective treatment. TB disease is a reportable condition in all U.S. jurisdictions; however, speciation of M. tuberculosis complex is not reported nationally. Approximately 80% of cases in the United States are culture confirmed. Systematic speciation was not feasible until the advent of comprehensive genotyping. M. bovis also can be distinguished from other species of M. tuberculosis complex by its pyrazinamide resistance and by biochemical tests available in reference laboratories; genetic deletion analysis identifies M. bovis definitively. The CDC national genotyping program for TB isolates incorporates spoligotype and MIRU, with IS6110 RFLP upon special request. However, RFLP is poorly discriminatory for M. bovis because isolates usually have a low number of IS6110 copies. Spoligotype variability among M. bovis isolates from the same cattle herd and similar spoligotype patterns from cattle in different regions have been observed (7). MIRU can yield more patterns than RFLP (8). PGRS has been recommended as the method of choice for strain typing of isolates with low copy numbers of IS6110 (9); however, in the NYC investigation, PGRS did not further differentiate clusters among the cases. The matching genotypes that defined the cluster of 13 cases might imply a transmission linkage; however, the significance of genotype clustering among M. bovis isolates is undetermined. The ongoing investigation in NYC has determined that human-to-human transmission was an unlikely explanation. New York and surrounding states are accredited as TB free for M. bovis in cattle§. Cow's milk products approved for sale in New York state are pasteurized with a few regulated exceptions¶. In contrast, a previous study determined that 17% of cattle sampled at meat-processing plants in Mexico were infected with M. bovis (10). An estimated 20% of cow's milk in Mexico destined for production of fresh cheese and similar products is not pasteurized. Other pathogens potentially acquired by consuming unpasteurized cow's milk products include Listeria monocytogenes, Salmonella spp., Brucella spp., Staphylococcus aureus, and Escherichia coli. To prevent infections with these bacteria, consumption of unpasteurized cow's milk products should be avoided**. References
* The investigation is led by the NYC Department of Health and Mental Hygiene, in collaboration with the New York State Department of Agriculture and Markets, CDC, the U.S. Department of Agriculture, and the Food and Drug Administration. † The Food and Drug Administration permits sale of imported or domestic, aged cheeses from unpasteurized milk under certain conditions. (Cheeses and related cheese products, 21 C.F.R. Part 133 [2005]). § Accredited-free states or zones, 9 C.F.R. Sect. 77.7 (2003). ¶ New York Codes, Rules, and Regulations. Title 1, Department of Agriculture and Markets; chapter I, milk control; subchapter A, dairy products; part 2, requirement for the production, processing, manufacturing, and distribution of milk and milk products. ** 21 C.F.R. Part 133 (2005).
Figure 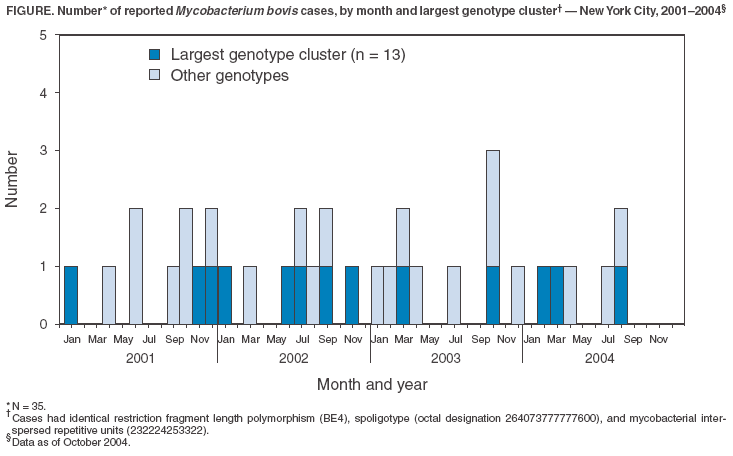 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 6/22/2005 |
|||||||||
This page last reviewed 6/22/2005
|