 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Estimated Influenza Vaccination Coverage Among Adults and Children --- United States, September 1--November 30, 2004Because of the unexpected reduction in the amount of available inactivated influenza vaccine for the 2004--05 influenza season, on October 5, 2004, the Advisory Committee on Immunization Practices (ACIP) recommended that the vaccine be reserved for persons in certain priority groups and asked others to defer or forego vaccination (1). To assess the use of influenza vaccine and the primary reasons reported for not receiving vaccine, beginning November 1, questions were added to the ongoing Behavioral Risk Factor Surveillance System (BRFSS) survey. This report analyzes data collected during December 1--11 on self-reported vaccination during September 1--November 30, which indicated that persons in nonpriority groups had largely deferred vaccination and that, among unvaccinated adults in priority groups, one fourth tried to get vaccine but were unable to do so. Vaccination coverage was suboptimal for persons in all assessed priority groups. Because influenza activity peaks in February or later in most years (2), persons in priority groups should continue to seek vaccination. BRFSS is a monthly, state-based, random-digit--dialed telephone survey of the U.S. civilian, noninstitutionalized population aged >18 years, with an average of 20,000 completed surveys per month (3,4). In previous influenza seasons, the BRFSS survey included two questions on influenza vaccination coverage among adults: "During the past 12 months, have you had a flu shot?" and "During the past 12 months, have you had a flu vaccine that was sprayed in your nose?" Questions on health-risk status were limited, and no information was collected on the timing of vaccination or on influenza vaccination among children. Beginning November 1, the two influenza vaccination questions were also asked regarding a randomly selected child in the household. In addition, new questions for adults and children were asked to determine 1) the month and year of the most recent influenza vaccination, 2) whether persons were vaccinated for influenza during the 2003--04 influenza season, 3) the primary reason vaccination was not received, and 4) whether the respondent (or a child in the household) was in one of the following ACIP-designated priority groups for vaccination: persons aged >65 years or aged 6--23 months, persons aged 2--64 years with one or more conditions that increase risk for influenza complications, health-care workers with patient contact, and household contacts of children aged <6 months*. For adults, conditions considered as increasing risk for influenza complications were asthma, other lung problems, heart problems, diabetes, kidney problems, weakened immune system, anemia, and pregnancy. For children, these conditions (with the exception of pregnancy) and aspirin therapy were considered as increasing risk for influenza complications. Children aged 6 months--8 years are recommended to have 2 doses of influenza vaccine if they have never been vaccinated for influenza (2). However, in this survey, assessment of 1 versus 2 doses was not made, and children were counted among those vaccinated if they received any influenza vaccination. The analyses were based on 16,713 interviews conducted during December 1--11 and thus represent partial influenza season estimates. Data were available for 48 states and the District of Columbia; data for Nevada and New Mexico were not available. Because BRFSS data collection is ongoing, response rates for December are not yet available. The median response rate for states/areas for the preceding month (November 2004) was 52.3% (range: 23.2%--76.8%) based on CASRO guidelines. For 2003, the last year for which yearly response rates are available, the median response rate for states/areas was 53.2% (range: 34.4%--80.5%). Although response rates have declined over time, when BRFSS data are compared with census data and other surveys, BRFSS data have a minimal bias and are reliable (3,4). Estimates were adjusted to account for differential probabilities in the sample selection, the age- and sex-specific population from the 2003 census for each state, and the size of the state population. Statistical analysis software was used to account for the complex sampling design and to calculate standard errors and confidence intervals. Vaccination Coverage Among AdultsAmong adults in all priority groups, 34.8% reported receiving an influenza vaccination during September 1--November 30, compared with 4.4% of adults aged 18--64 years who were not in a priority group (Table 1). Coverage was highest (51.1%) among persons aged >65 years, followed by health-care workers with patient contact (34.2%) and adults aged 18--64 years with high-risk conditions (19.3%). The percentage of persons reporting that they obtained an influenza vaccination during September 1--November 30 was smaller in each of these groups than the percentage who said they obtained a vaccination during the previous influenza season, September 1, 2003--March 31, 2004. Among persons aged >65 years who reported receiving influenza vaccine during the 2003--04 influenza season, 71.7% reported also being vaccinated during the 2004--05 influenza season. State-specific, self-reported vaccination coverage among adults in priority groups ranged from 18.0% to 60.3%, with a median of 37.6% (Figure). Among all vaccinated adults, 1.6% reported receiving FluMist®, the live attenuated influenza vaccine (LAIV) approved for use by healthy persons aged 5--49 years who are not pregnant and not contacts of severely immunocompromised persons. Among adults in priority groups who had not yet received influenza vaccine, 23.3% reported that they attempted to obtain vaccination but could not; among persons aged >65 years, the proportion was 32.5% (Table 2). Among adults not in a priority group who had not received vaccine, 10.4% reported that they attempted to obtain vaccination but could not. Among adults in priority groups, 10.0% of adults said they were saving the vaccine for others, and 6.5% thought that they were not eligible to receive the vaccine. Vaccination Coverage Among ChildrenA substantially greater proportion of children in priority groups received at least one influenza vaccination this season compared with other children; 36.6% of children aged 6--23 months and 26.8% of children aged 2--17 years with high-risk conditions were vaccinated, compared with 8.9% of children aged 2--17 years with no high-risk condition (Table 3). Among those children aged 2--17 years with high-risk conditions who were vaccinated for influenza during the 2003--04 influenza season, 51.6% also have been vaccinated thus far this season. Among respondents with an unvaccinated child aged 6--23 months, 62.9% reported that they thought the vaccine was not needed, 8.4% reported that they tried but could not obtain vaccination for the child, 1.0% thought the child was ineligible for influenza vaccination, and 0.3% said they were saving the vaccine for those who needed it (Table 4). For respondents with an unvaccinated child aged 2--17 years with a high-risk condition, 38.4% reported that they thought vaccination was not needed, 14.4% reported that they tried but could not obtain vaccination, 12.5% thought their child was not eligible, and 10.3% said they were saving the vaccine for others. Reported by: MW Link, PhD, AH Mokdad, PhD, L Elam-Evans, PhD, LS Balluz, PhD, WS Garvin, WP Bartoli, GM Town, MS, M Sussman-Walsh, K O'Neill, D Gilbertz, Div of Adult and Community Health, National Center for Chronic Disease Prevention and Health Promotion; SY Chu, PhD, Office of the Director; GL Euler, DrPH, CJ Brown, MS, PJ Lu, PhD, CB Bridges, MD, Epidemiology and Surveillance Div; S Stokley, MPH, Immunization Svcs Div, National Immunization Program, CDC. Editorial Note:Influenza vaccination coverage data from the period September 1--November 30 suggest that persons in influenza vaccine priority groups are receiving vaccine at higher rates than persons in nonpriority groups at this point in the 2004--05 season. However, these early estimates of vaccine coverage among priority groups are below the vaccination rates for the full 2003--04 season for these groups. Efforts to vaccinate these persons should continue as vaccine becomes available. Data from the 2003 National Immunization Survey (NIS) indicate that vaccination coverage among children aged 6--23 months for the 2002--03 influenza season was substantially lower (7.4%) than the 2004--05 partial season coverage indicated by BRFSS data (36.6%) (5). In 2002, ACIP first encouraged influenza vaccination of children aged 6--23 months and close contacts of children aged <2 years, when feasible. In April 2004, ACIP strengthened that encouragement into a recommendation that all children aged 6--23 months be vaccinated annually for influenza (2). However, the majority (62.6%) of respondents with unvaccinated children aged 6--23 months did not think vaccination was needed for those children, indicating that further efforts are needed to educate the public about the new influenza vaccination recommendation for young children. The findings in this report are subject to at least four limitations. First, BRFSS is a land-line telephone-based survey and excludes those segments of the population without telephones or who use only cellular telephones. Second, data are self reported and subject to recall bias, particularly for questions that require recall over a longer period; therefore, for certain behaviors, prevalence estimates might be under- or overestimated. Third, certain influenza vaccine priority groups were not considered in the survey, including institutionalized adults and adult caretakers of children aged <6 months outside of the home (e.g., child care workers). Finally, because interviewing is not yet completed for December, these estimates might be subject to nonresponse bias if the responses from those who will be interviewed later in the month differ substantially from the results in this report. However, these vaccination coverage estimates are higher than estimates from BRFSS data collected in November and are consistent with public health messages encouraging those in priority groups to seek vaccination and asking others to forego vaccination. Estimates from BRFSS data of vaccination coverage for certain priority groups differ from those obtained by the influenza survey of the Harvard School of Public Health (HSPH), also published in this issue (6). The methodologies used in these surveys differ in at least three important respects, which might contribute to the differences in results. First, the interviews were conducted at different times and provide estimates of vaccination coverage at different points in the 2004--05 influenza season. BRFSS was conducted during December 1--11; the HSPH survey was conducted during October 29--November 9. Second, BRFSS data were collected individually by 48 states and the District of Columbia and reflect the combined responses of more than 16,713 adults; the HSPH survey was a national survey of 1,227 adults. Finally, the two surveys differed in how they measured the impact of the vaccine shortage on vaccination coverage. BRFSS asked a single, open-ended question of adults and one of adults residing with children to assess the primary reason persons had not received vaccination as of the date of interview. HSPH used a more extensive series of questions to assess the impact of the shortage. Influenza vaccination coverage estimates from this survey, when applied to U.S. population estimates for each of the priority and nonpriority groups, indicate that an estimated 45 million doses of influenza vaccine had been administered to noninstitutionalized persons as of November 30; approximately 73% of these doses were obtained by persons in priority groups. An estimated 58 million doses of inactivated vaccine and up to 3 million doses of LAIV are expected to be available for the United States for this influenza season. Thus, adequate doses of vaccine appear to remain to meet the anticipated demand among priority groups for influenza vaccination, based on 2003--04 coverage estimates from this survey. Although the survey did not assess coverage among institutionalized persons in priority groups, this projection also suggests that vaccine should be available to meet the demand of the nation's approximately 1.5 million nursing home residents. In addition, use of LAIV is an option for the vaccination of persons in certain priority groups (e.g., health-care workers who do not work with severely immuno-compromised persons and household contacts of children aged <6 months). LAIV is approved by the Food and Drug Administration for use among healthy persons aged 5--49 years who are not pregnant. Geographic differences in vaccine distribution and demand exist. To ensure that all available vaccine is used, state or local public health officials who determine that all persons in priority groups seeking vaccine have received vaccination and additional vaccine remains on hand might choose to recommend limited expansion of vaccination eligibility in their areas. Such expansion might include persons aged 50--65 years, household contacts of persons in priority groups, or other populations considered at increased risk by state or local officials. However, even if such a recommendation is made, private providers with large volumes of unused vaccine should, wherever practical, work with the state to transfer these doses to other states with unmet needs among persons in the ACIP priority groups. CDC continues to work with manufacturers, distributors, and state immunization programs to distribute vaccine to those states with unmet demand among the priority groups. Until the demand for vaccine has been met for all persons in ACIP priority groups in all states, vaccine held in the public sector should continue to be shared with those states whose vaccine supply is not sufficient to cover their priority groups. Persons with questions regarding influenza vaccine availability should contact their state and local health departments. References
* Certain additional priority groups cited by ACIP were not included in the survey, including residents of nursing homes and long-term--care facilities, out-of-home caregivers for children aged <6 months, and child household contacts of children aged <6 months.
Table 1 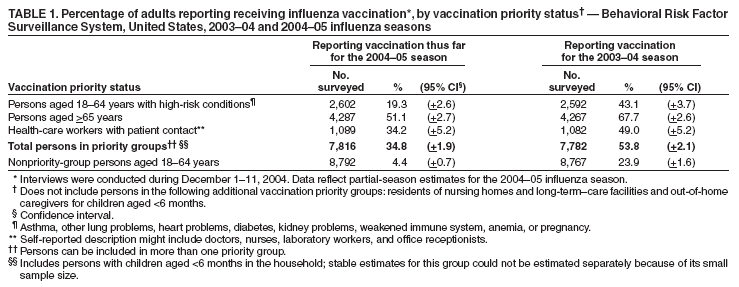 Return to top. Table 2 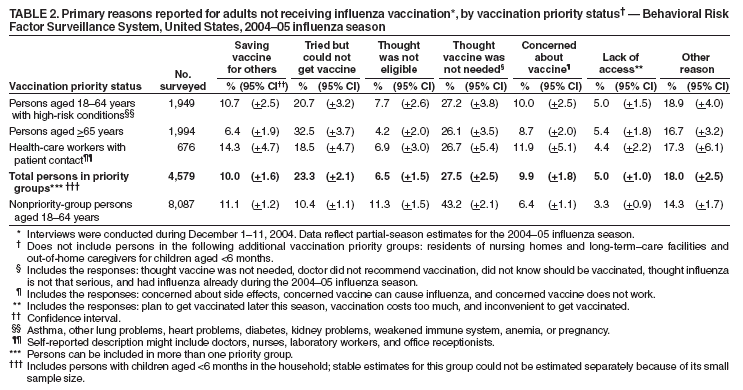 Return to top. Table 3 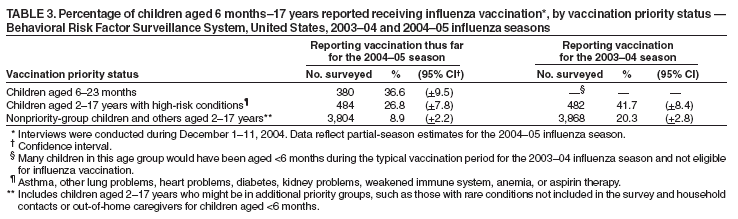 Return to top. Table 4 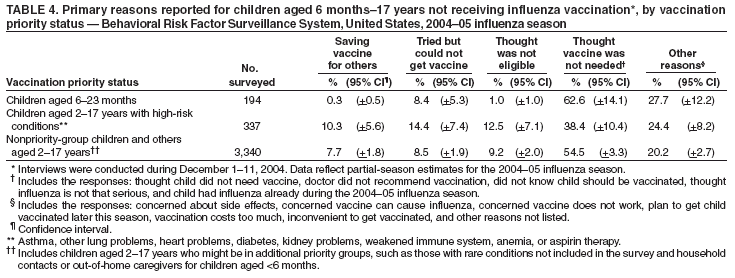 Return to top. Figure 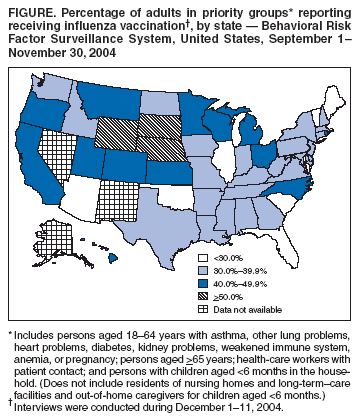 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/16/2004 |
|||||||||
This page last reviewed 12/16/2004
|