Key points
- Legionnaires’ disease clusters and outbreaks can present unique challenges for state and local public health laboratories.
- Laboratory staff can increase their preparedness by developing a Legionnaires’ disease Laboratory Response Plan (LDLRP).
- CDC designed this toolkit to help laboratory staff and leadership prepare an effective response plan.
Scope of the toolkit
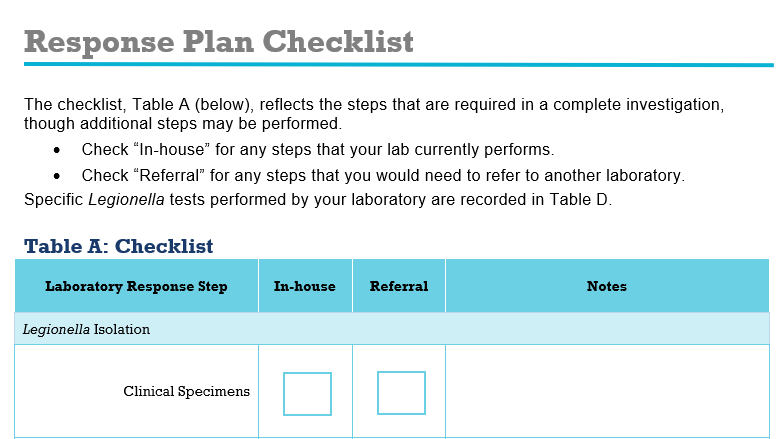
This toolkit provides a roadmap for public health laboratories to prepare for in-house work and referral plans ahead of an investigation.
The toolkit includes multiple templates, examples, and samples of the various planning tools.
Templates
- Templates for response team roles and responsibilities
- Planning templates for testing clinical specimens and environmental samples
- Planning templates for referring samples to an outside laboratory
Examples and samples
- A checklist to assess current Legionella testing capacity
- Example response scenario with sample workflow and timeline
- Example instructions for specimen storage and shipping
- Sample LDLRP
- Sample worksheets to document laboratory results
Details
Background
Legionnaires' disease is a serious type of pneumonia caused by the bacterium Legionella. When a Legionnaires' disease cluster or outbreak occurs, public health laboratories play a vital role in the response.
The process of culturing and analyzing Legionella environmental samples and clinical specimens can become complex. However, the process is often critical to determining where the patient(s) may have been exposed to Legionella.
