About
The CDC Specimen Test Order and Reporting Web Portal (CSTOR) Original Submitter Onboarding functionality, or COSO, allows non-state and non-federal level organizations (i.e., hospitals, local or county public laboratories, commercial laboratories, veterinary clinics, universities, etc.) to onboard to CSTOR as original submitters and submit specimens to CDC with associated State Public Health Laboratory (SPHL) visibility and pre-approval. These health organizations currently submit specimens to CDC for testing via the 50.34 Specimen Submission Form as ‘original submitters’ or ‘intermediate submitters.’ COSO streamlines the specimen submission workflow and ultimately provides SPHLs with greater visibility into submissions in their jurisdiction going to the CDC for testing.
Benefits
COSO has a wide range of benefits for CDC and external partners, including faster, more secure specimen submission and electronically tracked test order request approvals and rejections in CSTOR. Other benefits include:
- For Original Submitters: COSO offers a faster, streamlined workflow to receive SPHL approval and to submit specimens to CDC through Secure Access Management Services (SAMS) and the ability to correct specimen data before sample submission to mitigate sample rejections.
- For SPHLs: Provides more visibility into submissions from original submitters in respective jurisdictions, streamlining CDC report distribution. Also enables jurisdictions to review specimen submission forms prior to submission and SPHLs to request feedback and data correction prior to submission.
- For CDC: COSO promotes higher quality, streamlined, and more secure data submissions from health partners and providers.
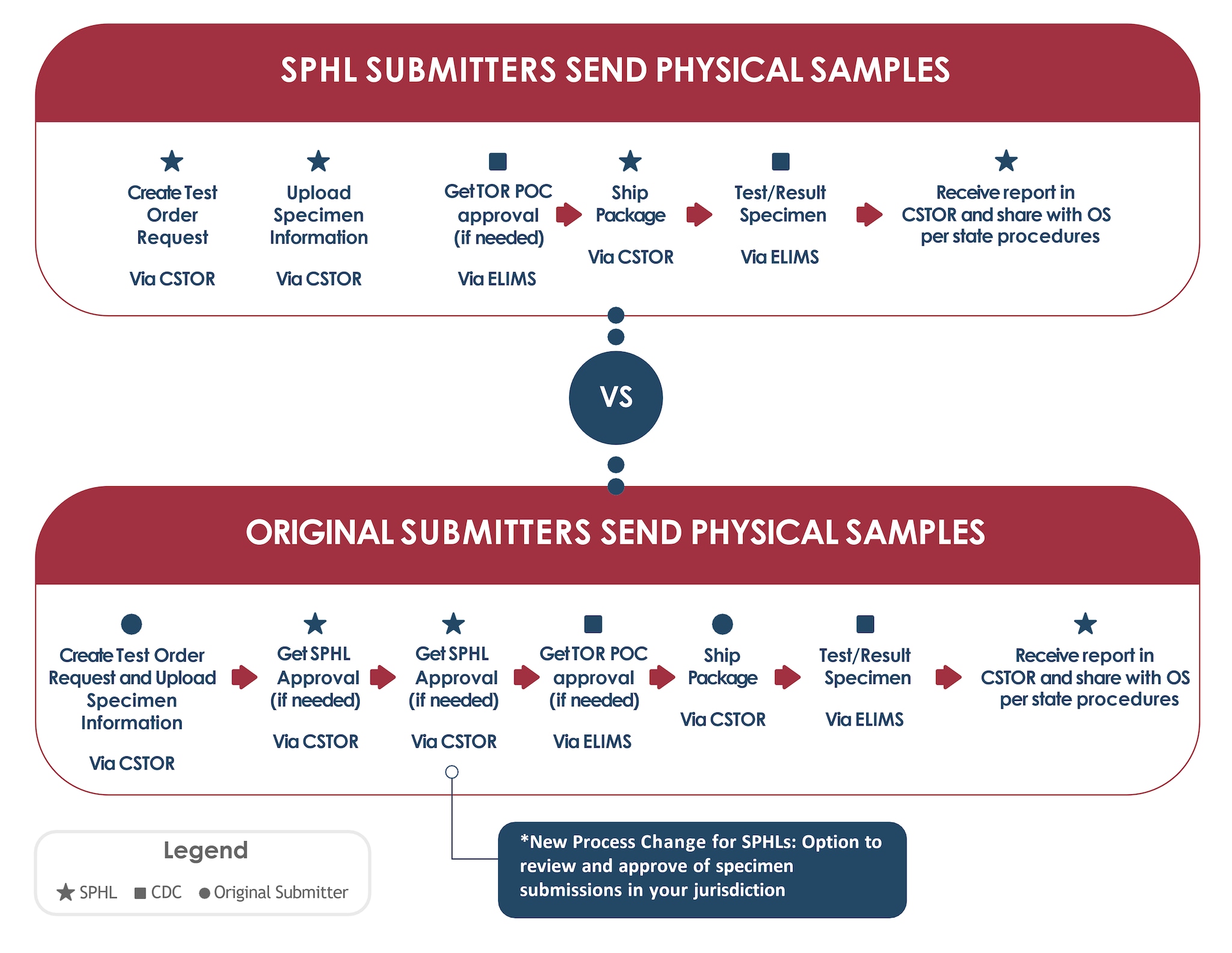
Onboarding to CSTOR as an original submitter
Any health organization that currently submits specimens to CDC for testing via the CDC 50.34 Specimen Submission Form as an 'original' or 'intermediate' submitter may be eligible for CSTOR onboarding. Please note the SPHL in the organization's jurisdiction will receive the request to onboard and is responsible for approving the request. We encourage coordination between SPHLs and original submitters prior to submitting the request to onboard to CSTOR.
For more information about CSTOR and how to onboard, please refer to the CSTOR Web Portal page. Original submitters who would like to onboard can fill out the CSTOR Original Submitter Onboarding REDCap form.
A streamlined CDC specimen submission process
- With SPHL approval, original submitters will first onboard to CSTOR via a secure form on REDCap. SPHLs will review and approve or reject the onboarding request. If approved, original submitters will receive instructions on how to onboard via SAMS.
- Once onboarded, when specimens are ready to ship to CDC, original submitters will create a Test Order Request (TOR) and upload specimen information in CSTOR.
- The associated SPHL will then review and approve or reject the submission (excluding test orders that have been auto-approved by the SPHL). If approved, the CDC will then initiate the review/approval process if the TOR requires CDC pre-approval. Once approved, the original submitter ships the specimen submission package using the shipping label created in CSTOR.
- Once received by the CDC, the specimens are tested in the appropriate CDC Infectious Disease (ID) laboratory and SPHLs can access and view the report(s) in CSTOR.
- Original submitters will receive reports from their SPHL.