About
- HPV-IMPACT enables CDC to monitor trends in cervical lesions that could progress to cancer and the reduction of cancer-causing HPV types that can be prevented by HPV vaccine.
- These are ways we can use to measure the impact of the HPV vaccine.
Purpose
Started in 2008, the Human Papillomavirus (HPV) Vaccine Impact Monitoring Project (HPV-IMPACT) monitors rates of high-grade cervical lesions in the United States. Scientists use data from the project to determine the impact of the U.S. HPV vaccination program on cervical precancers caused by HPV. High-grade cervical lesions are precancers that could progress to invasive cervical cancer if left untreated.
CDC uses the data from HPV-IMPACT to describe trends in high-grade cervical lesions and the reduction of cancer-causing HPV types (such as HPV16 and HPV18) in people diagnosed with these cervical lesions. Monitoring high-grade cervical lesions and the reduction of the HPV types that are prevented by vaccination are ways to measure the impact of the HPV vaccine.
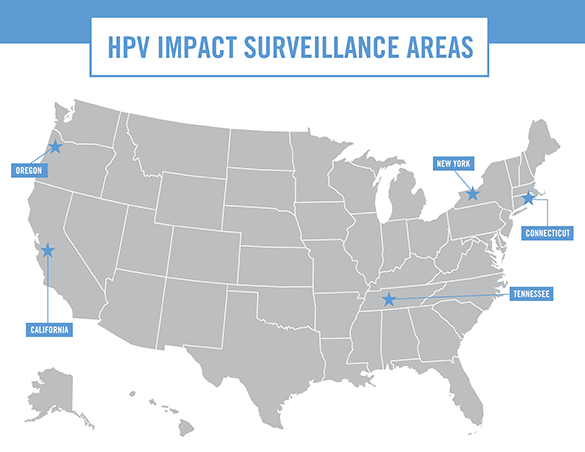
This surveillance project, monitors cervical lesions among adults ages 18 and older in five different communities around the United States.
- We collect demographic and clinical information from laboratory and medical records for every woman who has a high-grade cervical lesion identified.
- For people aged 18-39 years, we also collect information such as insurance, cervical cancer screening history, and vaccination history.
- We obtain archived tissue specimens for HPV type testing for a panel of HPV types, including those targeted by HPV vaccines.
The main objectives of HPV-IMPACT are to:
- Monitor trends in overall incidence of high-grade cervical lesions (CIN2+) over time in defined populations;
- Monitor prevalence and distribution of HPV genotypes in 18–39-year-olds with CIN2+;
- Describe the demographic, clinical, and HPV type characteristics among patients with CIN2+;
- Estimate and monitor trends in cervical cancer screening utilization among residents of the catchment areas; and
- Estimate the proportion of patients with CIN2+ who received the HPV vaccine, and estimate vaccine effectiveness.
How we collect data
The HPV-IMPACT network conducts surveillance of over 1.5 million women ages 18 and older in five states, including enhanced surveillance among over half a million 18–39-year-olds. The HPV-IMPACT surveillance areas include Monroe County, NY; Davidson County, TN; New Haven County, CT; Alameda County, CA; and 28-zip codes in metropolitan Portland, OR, including portions of Multnomah County and Washington County. The surveillance sites include racially and ethnically diverse populations similar to the United States as a whole.
The table below identifies the state partners participating in HPV-IMPACT.
What's included
Since 2008, HPV-IMPACT has monitored pre-invasive cervical lesions that could progress to invasive cancer if left untreated as early indicators of population vaccine impact. Because showing the impact of vaccine on reducing the burden of cervical cancer is of highest importance, we revised the case definition to include invasive cervical cancer. Cervical carcinomas diagnosed in residents of the catchment area are identified retrospectively (2008-present). Tissue for HPV typing is requested for cervical carcinoma cases diagnosed in 2016 or later. Once all sites have submitted data on cancer cases, reporting of CIN2+ case counts will include cancers.
Case Definition
A case is defined as a resident in one of the surveillance areas who has a histologically confirmed diagnosis of cervical intraepithelial neoplasia (CIN) grades 2 or 3, or adenocarcinoma in situ (collectively referred to as CIN2+) and is at least 18 years of age at the time of diagnosis. The date of diagnosis must be on or after January 1, 2008.
Case Identification
Cases are identified based on laboratory reports of CIN2+. Each laboratory in the surveillance area regularly provides laboratory reports of CIN2+ findings to the local site. Because classification for pre-invasive cervical neoplasia is not standardized in the United States, a master list of possible diagnostic codes, terminology, and search terms has been provided to all sites and reporting laboratories to standardize case identification.
Data Collection Methods
For each case of CIN2+ identified among patients aged 18 years or older, a case report form with basic demographic and clinical data is completed. If the patient is between the ages of 18-39 years, an enhanced case report form is completed and tissue specimen is sent to CDC for HPV type testing. Additional data include race, ethnicity, insurance, cervical cancer screening history, and vaccination history and are obtained from laboratory and medical records.
Laboratory Methods
A portion of tissue that was originally used by a pathologist to diagnose the patient with CIN2+ is sent to CDC for HPV DNA typing. A pathologist at CDC examines tissue samples to make sure that a high-grade cervical lesion is present in the tissue specimen that was sent. DNA is extracted and tested for a panel of HPV types, including all vaccine types and several additional high-risk and non-high-risk HPV types. From 2008-2019, all specimens were tested for 37 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, IS39). During 2020, the HPV laboratory is transitioning to using a new assay that will detect 28 HPV types. The previously used assay has been discontinued by the manufacturer.
