At a glance
The technical notes provide information on how the data in the Community Counts Data Visualization are collected and displayed.
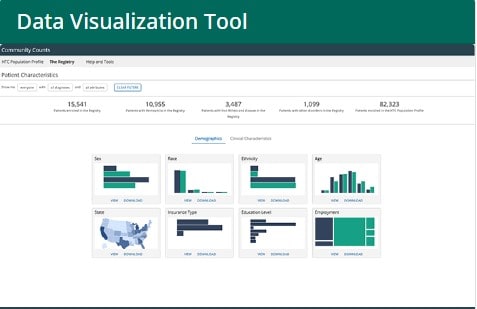
What is the Community Counts Data Visualization Tool?
Click here to print a PDF of the technical notes.
What is Community Counts?
The Community Counts Public Health Surveillance of Bleeding Disorders project (Community Counts) is a public health monitoring program. The purpose of Community Counts is to gather and share information about health issues, medical complications, and causes of death that affect people with bleeding disorders who receive care at federally funded US Hemophilia Treatment Centers (HTCs).
What Community Counts data are displayed in the Data Visualization Tool?
The Community Counts Data Visualization Tool displays de-identified data on people with bleeding disorders who are enrolled in Community Counts in an interactive, visual format. The data are updated monthly. The data are subject to revision and may change over time.
Two components of Community Counts are displayed:
- The HTC Population Profile (HTC PP), which gathers basic information on all people with bleeding disorders or blood clots who receive care at HTCs.
- The Registry for Bleeding Disorders Surveillance (Registry), which gathers more detailed information on a subset of HTC PP participants with bleeding disorders who volunteer to participate and have their information collected.
The next sections provide more information about the HTC PP and Registry and describe the data displayed in the visualizationA.
HTC PP
Who collects the data? HTC staff collect and report information on HTC PP participants. The information is collected through observation, from participants' medical records, and collected from the participant as part of their clinical care. Standardized forms are used to collect the information.
When are the data collected? HTC PP data are collected each year from all federally funded HTCs. HTCs are asked to provide data for all participants who came in for an annual visit.
What data are displayed? The HTC PP data in the visualization are from unique participants who have attended an HTC at least once since January 2012. The data in the graphs are from participants' most recent annual visit. In contrast, the Registry data that are displayed (see below) are from participants' enrollment visits. Categories with fewer than five participants are suppressed (that is, not shown in the data visualization) to protect participant confidentiality. Additional counts may be suppressed to prevent calculation of these counts by subtraction.
The following types of information are displayed in the visualizationB:
- Demographic information (for example, sex, race, ethnicity, and age);
- Primary bleeding or clotting disorder diagnosis; and
- Human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infection status.
Registry
Who collects the data? HTC staff collect and report information on Registry participants. The information is collected through observation, from participants' medical records, and collected from the participant as part of their clinical care. Standardized forms are used to collect the information.
When are the data collected? Data from persons who participate in the Registry are collected multiple times—at their initial (enrollment) visits to the HTC and thereafter at their annual visits. HTC staff record information on a participant's health history as well as their current health information on the enrollment visit form. Thereafter, the subsequent visit form is used to collect health information since the previous visit.
What data are displayed? The Registry data in the visualization are from unique participants enrolled since December 2013. The Registry data in the graphs are from participants' enrollment visits. In contrast, the HTC PP data are from participants' most recent annual visit. Categories with fewer than five participants are suppressed (that is, not shown in the data visualization) to protect participant confidentiality. Additional counts may be suppressed to prevent derivation of these counts by subtraction.
Some of the information collected for Registry participants is the same as that collected for the HTC PP (for example, demographics, bleeding disorder diagnosis, and HIV and HCV infection status). The additional Registry information below is displayed in the visualization:
- More detailed demographics (for example, education and employment status)
- Primary residence
- Insurance type
- Vaccination status
- Weight and height
- Treatment regimen
- Treatment products
- Age at first treatment for bleeding and when prophylaxis started
- Location of care
- Hospitalization information (for example, number of inpatient nights and absenteeism)
- Other medical conditions (that is, in addition to the person's bleeding or clotting disorder)
- Chronic pain
Patient Characteristics
Below are the technical notes for each data element presented in graphs in the Patient Characteristics–Demographics module. Some data are collected for both the HTC PP and the Registry, while other data are only collected for the Registry. For each data element, the technical notes describe the information provided to HTC staff who fill out the standardized forms for each participant.
Sex (graph displays HTC PP and Registry data)
- Male
- Female
Race (graph displays HTC PP and Registry data)
Race is displayed as
- White: A person having origins in any of the original people of Europe, the Middle East, or North Africa.
- Black or African American: A person having origins in any of the Black racial groups of Africa.
- Other races: Includes people who are American Indian/Alaska Native, Asian, or Native Hawaiian or other Pacific Islander.
- More than one race or unknown: Includes people who belong to more than one racial group or people for which no race information was provided.
Ethnicity (graph displays HTC PP and Registry data)
Ethnicity is displayed as
- Hispanic, Latino/a, or Spanish Origin: Includes people of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin, regardless of race.
- Not Hispanic, Latino/a, or Spanish Origin or Unknown: Includes people who are not of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin, or people for which no ethnicity information was provided.
Age (graph displays HTC PP and Registry data)
For the HTC PP, year of visit and year of birth are collected (YYYY). Age is calculated by subtracting the year of birth from the year of visit. Although participants may be represented in multiple years of the HTC PP, age in this chart represents each unique participant's age at their most recent visit.
For the Registry, date of visit and date of birth are collected (MM/DD/YYYY). Age is calculated by subtracting the date of birth from the date of visit and dividing that number by 365 (leap years were accounted for in this calculation when applicable). Although participants may be represented in multiple years of the Registry, age in this chart represents each unique participant's age at their time of enrollment.
Because age is defined differently in the HTC PP and Registry, HTC PP ages change over time whereas Registry ages do not change. Also, because of the difference in definition, if you filter by age, the total count of people in the Registry may be higher than the total count of people in the HTC PP.
Both HTC PP and Registry ages are categorized as follows:
- <2 years
- 2–10 years
- 11–19 years
- 20–44 years
- 45–64 years
- 65+ years
Patient Geographic Information (graph displays Registry data only)
The five-digit ZIP code of the participant's primary residence at the time of enrollment is used to determine their state of residence. The HTC state in which a participant receives care is used for individuals who live on military bases, outside of the United States, had a missing ZIP code, or their ZIP code was reported as unknown.
Insurance Type (graph displays Registry data only)
Insurance is grouped into these categories based on the primary health insurance held by the participant.
- Private: Includes any type of commercial insurance provided through private or public companies and paid for by employers or by individuals. This includes military health care.
- Public: Includes Medicare, Medicaid, state-sponsored insurance plans, and Indian Health Services.
- Other/Uninsured/Unknown: Includes insurance not otherwise described as public or private (for example, single service plans paid for by the individual that provide coverage for only one type of service or treatment for a specific condition). This category also includes participants without insurance plan coverage for medical costs, and participants for whom it was not known whether they had any health insurance, or the type of health insurance was unknown.
Education Level (graph displays Registry data only)
Education is recorded as the highest level of formal education completed by the participant.
- None or pre-elementary: Includes participants with no formal education, including children too young to attend a formal education program and participants attending pre-kindergarten or kindergarten.
- Primary/secondary: Includes participants who completed grades 1–11.
- High school or equivalent: Includes participants who completed grade 12, the General Educational Development (GED) test, or equivalent.
- Some post-secondary: Includes participants who attended college but did not complete a degree; completed a 2-year degree program; or attended any post-high school or post-GED technical, vocational, or trade program.
- 4-year college degree (bachelor's): Includes participants who completed a 4-year program of study at a college or university.
- Advanced degree: Includes participants who completed a program of study at a college or university beyond the bachelor level, culminating in a master's, doctorate, or professional degree.
- Other or unknown: Includes participants with any other level of formal education that does not fall into one of the above categories and participants whose education level was not known.
Employment (graph displays Registry data only)
Employment status is displayed as the following categories:
- Employed full-time: Participants who were working 35 or more hours per week.
- Employed part-time: Participants who were working fewer than 35 hours per week.
- Not employed—child or student: Participants who were not working at all because they were a student or child. All participants younger than 18 years old are grouped into this category.
- Not employed—disabled: Participants who were not working and were known to have qualified for disability income and were therefore prohibited from working by law.
- Not employed—retired: Participants who were not working at all and were of retirement age (usually younger than 55 years old).
- Not employed—other or unknown: Participants who were homemakers and did not work outside of the home; participants who did not work and had no other listed reason for not working (this includes participants who are able to work but who have been laid off or cannot find work); and participants who did not work for a reason that is unknown, or known but not otherwise listed.
- Employment status unknown: participants whose employment status was unknown.
Clinical Characteristics
Below are the technical notes for each data element presented in graphs in the Patient Characteristics–Clinical Characteristics module. Some data are collected for both the HTC PP and the Registry, while other data are only collected for the Registry. For each data element, the technical notes describe the information provided to HTC staff who fill out the standardized forms for each participant. Information about how CDC calculated some of the data (for example, BMI) is also included.
Primary Diagnosis (graph displays HTC PP and Registry data)
Primary diagnosis is displayed for Registry and HTC PP participants. For persons with only one bleeding disorder diagnosis, the primary diagnosis is the same as their bleeding disorder diagnosis. For persons with more than one bleeding disorder diagnosis, primary diagnosis is assigned using the following framework:
- Primary diagnosis for participants who have hemophilia and another bleeding disorder is reported as hemophilia A (factor VIII [8] deficiency) or hemophilia B (factor IX [9] deficiency), as appropriate.
- Primary diagnosis for participants who have both factor VIII and factor IX deficiency is reported as whichever deficiency is more severe (has the lower baseline factor level); if equally severe, they are reported as hemophilia A.
- Primary diagnosis for participants who have von Willebrand disease (VWD) plus another bleeding disorder is reported as VWD (unless the other disorder is hemophilia, in which case the primary diagnosis is reported as hemophilia).
- Primary diagnosis for participants who have more than one other clotting factor deficiency is reported as whichever deficiency has the lowest baseline factor level.
- Primary diagnosis for participants who have a clotting factor deficiency and a platelet disorder, or a connective tissue disorder is reported as the clotting factor disorder.
- Hemophilia A: Includes participants diagnosed with factor VIII deficiency including carriers.
- Hemophilia B: Includes participants diagnosed with factor IX deficiency including carriers.
- VWD type 1: Includes participants diagnosed with VWD type 1 or type 1C.
- VWD type 2: Includes participants diagnosed with VWD type 2A, type 2B, type 2M, type 2N, or type 2, sub-type unknown.
- VWD type 3: Includes participants diagnosed with VWD type 3.
- VWD type other or unknown: Includes participants for whom VWD type is known, but is not one of the types listed above, or for whom VWD type was reported as unknown.
- Rare factor deficiencies: Includes participants with factor I (1) deficiency (including dysfibrinogenemia, hypofibrinogenemia, afibrinogenemia, and unspecified hereditary factor I [1] deficiency), factor II (2) deficiency, factor V (5) deficiency, combined factors V & VIII (5 & 8) deficiency, factor VII (7) deficiency, factor X (10) deficiency, factor XI (11) deficiency, factor XIII (13) deficiency, alpha-2 antiplasmin deficiency, or PAI-1 deficiency.
- Platelet disorders and other: Includes participants with gray platelet syndrome, Glanzmann thrombasthenia, Bernard-Soulier syndrome, Hermansky-Pudlak syndrome, inherited thrombocytopenia, platelet function disorder, platelet storage pool disease, platelet release defect or other hereditary functional platelet disorder, blood coagulation disorder with impaired clot retraction time, blood coagulation disorder with prolonged bleeding time, or blood coagulation disorder with prolonged coagulation time.
Severity of Hemophilia (graph displays HTC PP and Registry data)
Severity of hemophilia is displayed for Registry and HTC PP participants. Baseline clotting factor activity is used to determine hemophilia severity. Baseline clotting factor activity is used because it best represents participants' inherent clotting factor activity prior to receiving any treatment. HTC staff report each participant's baseline clotting factor activity level. Severity level is measured as a percentage and defined by the degree to which a person is deficient (lacking) in a clotting factor. For example, people with "severe hemophilia" have a baseline clotting factor activity level of less than 1%.
Due to technical limitations when displaying the data, the denominator for the percentages displayed in this graph is all participants, including people with non-hemophilia diagnoses. Thus, when you hover over a column, the percentage displayed is the number of people with a given severity of hemophilia (displayed in the pop-up window) divided by the total number of participants for the Registry or HTC PP.
To calculate the percentages of hemophilia severity categories for hemophilia participants only, follow these steps:
- Determine which hemophilia severity category you wish to calculate (mild, moderate, or severe) and which population of people you are interested in (Registry or HTC PP).
- Get the numerator by hovering over the desired column in the Severity of Hemophilia graph. The "count" is the numerator.
- Get the denominator for the Registry from the Patient Characteristics page, "Patients with Hemophilia in the Registry." Get the denominator for the HTC PP by clicking the Primary Diagnosis graph and adding together the number of people in the HTC PP with hemophilia A and B.
- Divide the number in step 2 (numerator) by the number in step 3 (denominator).
- Mild: Greater than 5% baseline clotting factor activity.
- Moderate: 1%–5% baseline clotting factor activity.
- Severe: Less than 1% baseline clotting factor activity.
- Unknown/Missing: Baseline clotting factor activity unknown or missing.
Viral Status (graph displays Registry data only)
History of hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) infection is displayed for Registry participants ages 18 years and older.
- HBV and HCV—Yes: Participant has been diagnosed with HBV or HCV infection, regardless of whether the infection has since resolved or if the participant is currently HBV or HCV viral load negative (there is no measurable level of HCV or HBV in their blood) or viral load positive (there is a measurable level of HCV or HBV in their blood).
- HIV—Yes: Participant has been diagnosed with HIV infection or acquired immunodeficiency syndrome (AIDS) during their lifetime.
- No: Participant has been tested but has not been diagnosed with HBV infection (active or resolved), HCV infection (active or resolved), or HIV infection or AIDS.
- Unknown/Not applicable: Includes participants for which results were unknown or unable to be determined; participants who have never been tested for HBV, HCV, or HIV; and participants younger than 18 years old.
Vaccination (graph displays Registry data only)
History of hepatitis A virus (HAV) vaccination reflects whether a participant reported (or their records indicated) a history of HAV vaccination (VAQTA®; HAVRIX®, TWINRIX®). History of HBV vaccination reflects whether a participant reported (or their records indicated) a history of HBV vaccination (Energix-B®, Recombivax HB®, TWINRIX®, Pediarix®, Comvax®).
- Yes: Participant's medical record indicated a history of vaccination or the participant (or the participant's parent, in the case of minors) reported vaccination.
- No: Participant's medical record indicated they had NOT been vaccinated, or the record did not address vaccination and the participant/parent reported that they had not been vaccinated.
- Unknown: Participant's record did not address vaccination status and the participant/parent did not know.
Weight Status (graph displays Registry data only)
Body mass index (BMI) was calculated for Registry participants ages 2 years and older using weight and height reported at enrollment. The formula used to calculate BMI is weight (kg)/[height] (m)]2. A corresponding weight status was assigned using established national guidelines, which are based on a person's BMI for adults,1 or age and BMI for children and teens.2
- Obese
- Overweight
- Normal
- Underweight
- Not applicable: Includes children younger than 2 years old.
Treatment
Below are the technical notes for each data element presented in graphs in the Treatment module. The data in these graphs are only from the Registry, as the information is not collected for the HTC PP. For each data element, the technical notes describe the information provided to HTC staff who fill out the standardized forms for each participant. Information about how CDC coded some of the data (for example, treatment products) is also included.
Treatment Regimen (graph displays Registry data only)
Treatment regimen is displayed as the following categories:
- Prophylaxis—Continuous with bypassing agents plus immune tolerance induction (ITI): participant used factor VIII (8) or factor IX (9) concentrates on a regular basis for the primary purpose of eliminating an inhibitor, and bypassing agents were used on a regular basis to prevent any and all bleeds (even if they did not follow the regimen perfectly), and the participant is expected to maintain this regimen indefinitely.
- Episodic (On-demand): Participant used treatment products to stop bleeding episodes only when they occurred.
- Prophylaxis—Continuous: Participant used treatment products on a regular basis to prevent bleeding episodes from occurring (even if they did not follow the regimen perfectly), and the participant is expected to maintain this regimen indefinitely.
- Prophylaxis—Event-based, short-term or intermittent: Participant used treatment products for the following situations: (1) To prevent anticipated bleeding episodes associated with an event (such as a dental procedure or surgery) or an activity (such as a sports event) on an intermittent basis. This routine could have been used repeatedly over a short period of time, or on a single occasion. (2) On a regular basis to prevent bleeding episodes for an extended period of time (weeks to months) but not indefinitely (such as only during a sports season).
- Prophylaxis—Menstrual bleeding: Participant used treatment products regularly to prevent excessive menstrual bleeding.
- Immune tolerance induction (ITI) without continuous prophylaxis with a bypassing agent: Participant used factor VIII or factor IX concentrates on a regular basis for the primary purpose of eliminating an inhibitor (even if they did not follow the regimen perfectly) and did not also use bypassing agents for continuous prophylaxis.
Treatment Products (graph displays Registry data only)
Treatment product names are reported on the visit form and CDC recoded these product names into the following grouped categories. The categories are not mutually exclusive; therefore, a participant may report more than one treatment product and therefore be counted in more than one category.
- Clotting factor concentrates: Includes recombinant, standard half-life products; recombinant, extended half-life products; and plasma-derived clotting factor concentrates.
- Antifibrinolytics: Includes aminocaproic acid and tranexamic acid.
- Desmopressin.
- Other treatment products: Includes novel products; bypassing agents; hormonal birth control/therapy; blood products; other product types (product[s] that do not fall into other categories listed; g., QR powder); and unknown (product[s] used were reported as unknown, only referred to the manufacturer name of a product, or were not described in sufficient detail to determine product category).
- No treatment products used.
Age at First Bleeding Treatment (graph displays Registry data only)
This reflects the age the participant was first treated for their bleeding disorder as evidenced by use of any source of clotting factor (clotting factor concentrates, plasma, or cryoprecipitate), Hemlibra®, desmopressin, aminocaproic acid, or tranexamic acid.
Age at first treatment for bleeding is calculated by subtracting date of birth (MM/DD/YYYY) from the date of first treatment for bleeding (MM/DD/YYYY) to get the age in days. Age in days is divided by 365 to get age in years (leap years were accounted for in this calculation when applicable). This data includes treatment dates that were actual and estimated (when the exact date of first treatment for bleeding could not be determined). When the actual or estimated treatment day was missing, the 15th of the month of treatment was used to create a whole date before performing subtraction. When the actual or estimated month and day of treatment were missing, June 15th and the year of treatment was used to create a whole date.
- <1 year
- 1 year
- 2–5 years
- 6–10 years
- 11–19 years
- 20–44 years
- 45+ years
- Unknown: the date of first treatment for bleeding could not be estimated to within a year of the event.
- Never treated: includes participants who have never received treatment for bleeding and participants who have never had a bleed.
Age Continuous Prophylaxis Started (graph displays Registry data only)
The age continuous prophylaxis started (in years) for participants on continuous prophylaxis, prophylaxis for menstrual bleeding, and continuous prophylaxis with bypassing agents plus ITI was calculated by subtracting date of birth (MM/DD/YYYY) from the date at which continuous prophylaxis or prophylaxis for menstrual bleeding was first started (either MM/DD/YYYY, MM/YYYY, or YYYY).
- <3 years
- 3–6 years
- >6 years
- Unknown: The date of first continuous prophylactic treatment could not be estimated.
- Not applicable: Participants who did not practice continuous prophylaxis, prophylaxis for menstrual bleeding, or continuous prophylaxis with bypassing agents plus ITI.
Plasma-Derived or Blood Product Used (graph displays Registry data only)
This graph displays whether participants used treatment products derived from plasma, whole blood, or blood components. Plasma-derived products are clotting factor concentrates derived from human plasma and bypassing agents derived from human plasma. Blood products are products other than plasma made from donated blood. Treatment product names are reported on the visit form and CDC coded them as plasma-derived or blood products.
- Yes: Participants used at least one treatment product that was plasma-derived, whole blood, or a blood component.
- No: Participants had not used any treatment product(s) that were plasma-derived, whole blood, or a blood component.
- Unknown: Product(s) used were reported as unknown or not described in sufficient detail to determine whether they were plasma-derived, whole blood, or a blood component.
Bleeding Disorders Burden
Below are the technical notes for each data element presented in graphs in the Bleeding Disorders Burden module. The data in these graphs are only from the Registry, as the information is not collected for the HTC PP. For each data element, the technical notes describe the information provided to HTC staff who fill out the standardized forms for each participant. Information about how CDC coded some of the data (for example, inpatient and ER visits) is also included.
Location of Care (graph displays Registry data only)
HTCs can provide care at their primary location, through an outreach clinic (when HTC staff go to a designated location to conduct participant visits), or through a telemedicine session. The locations are not mutually exclusive; a participant may have received care in more than one location and would be counted in more than one category.
- HTC primary location: Care was received at the primary HTC location.
- HTC outreach clinic: Care was received at a physical location to which HTC staff have traveled, distant from the HTC's primary location.
- HTC telemedicine session/clinic: Care was received from HTC staff remotely via a tele-connection.
Inpatient and ER Visits (graph displays Registry data only)
The number of hospital inpatient admissions and emergency room (ER) visits refers to visits made in the previous 12 months for care or management of a bleeding disorder or a complication thereof, including complications of HIV or hepatitis infection if the infection was due to the use of treatment products for the participant's bleeding disorder. If the precise number of visits was unknown, estimates could be provided or reported as unknown.
On the visit form, this is asked as two questions:
Question 1: "In the last 12 months, has the patient visited a hospital emergency room for a bleed or other complication of his/her bleeding disorder?" Response options are "Yes, No, and Unknown."
Question 2: If "yes," "How many emergency room visits did the patient have due to his/her bleeding disorder in the last 12 months?" Response option is to write in a number value or select "Unknown."
CDC re-coded these two questions into one variable as follows:
- If the answer to question 1 was "Yes," the number of visits were re-coded to the appropriate grouped category, or re-coded to "Unknown" if applicable.
- If the answer to question 1 was "No," this was re-coded to "0 visits."
- If the answer to question 1 was "Unknown," this was coded as "Unknown."
- 0: Indicates no inpatient admissions or trips to the ER in the previous 12 months.
- 1: Indicates 1 inpatient admission or trip to the ER in the previous 12 months.
- 2–3: Indicates 2 or 3 inpatient admissions or trips to the ER in the previous 12 months.
- 4–8: Indicates 4, 5, 6, 7, or 8 inpatient admissions or trips to the ER in the previous 12 months.
- 9+: Indicates 9 or more inpatient admissions or trips to the ER in the previous 12 months.
- Unknown: It was unknown whether the participant had visited the ER for care of their bleeding disorder or how many inpatient/ER visits they had in the previous 12 months.
Inpatient Nights (graph displays Registry data only)
The number of inpatient nights refers to the number of nights spent as an inpatient in the previous 12 months for participants who reported an inpatient admission. The inpatient nights were for care or treatment of a bleeding disorder or a complication or anticipated complication (preventive hospitalization) thereof, including complications of HIV or hepatitis infection if the infection was due to use of treatment products for the participant's bleeding disorder.
On the visit form, this is asked as two questions:
Question 1: "In the last 12 months, has the patient been admitted to a hospital as an inpatient due to a bleed or other complication of his/her bleeding disorder?" Response options are "Yes, No, and Unknown."
Question 2 If "yes," "How many nights total did the patient spend as an inpatient due to his/her bleeding disorder in the last 12 months?" Response option is to write in a number value or select "Unknown."
CDC re-coded these two questions into one variable as follows:
- If the answer to question 1 was "Yes," the number of visits were re-coded to the appropriate grouped category, or re-coded to "Unknown" if applicable.
- If the answer to question 1 was "No," this was re-coded to "0 visits."
- If the answer to question 1 was "Unknown," this was coded as "Unknown."
- 0: Indicates no inpatient nights in the previous 12 months.
- 1: Indicates 1 inpatient night in the previous 12 months.
- 2–3: Indicates 2 or 3 inpatient nights in the previous 12 months.
- 4–8: Indicates 4, 5, 6, 7, or 8 inpatient nights in the previous 12 months.
- 9+: Indicates 9 or more inpatient nights in the previous 12 months.
- Unknown: It was unknown whether the participant had been admitted to a hospital overnight as an inpatient for care of their bleeding disorder or how many nights they had spent as an inpatient in the previous 12 months.
Absenteeism (graph displays Registry data only)
Absenteeism refers to the numbers of days missed from school or work in the previous 12 months because one's bleeding disorder impacted their normal activities to the extent that they could not attend work or school.
- 0: No days missed in the previous 12 months.
- 1–2: Indicates 1 or 2 days missed in the previous 12 months.
- 3–4: Indicates 3 or 4 days missed in the previous 12 months.
- 5–10: Indicates 5, 6, 7, 8, 9, or 10 days missed in the previous 12 months.
- 11+: Indicates 11 or more days missed in the previous 12 months.
- Unknown: Indicates the number of days missed from school or work in the previous 12 months was unknown.
- Not applicable: Indicates the participant did not attend school or work.
Chronic Pain (graph displays Registry data only)
Chronic pain refers to pain that has been present for 3 months or longer and is related to a participant's bleeding disorder. Chronic pain is reported as Yes, No, or Unknown. For participants who reported "Yes" to experiencing chronic pain in the previous 12 months, the frequency of chronic pain is also recorded.
Yes: Participant had experienced pain related to his/her bleeding disorder.
- Frequency of chronic pain: Every day, Most days, Some days, Unknown
No: Participant had not experienced chronic pain related to his/her bleeding disorder.
Unknown: It was unknown whether the participant had experienced chronic pain related to his/her bleeding disorder.
Opioid Use for Chronic Pain (graph displays Registry data only)
Opioid use for chronic pain is only reported for individuals who responded "Yes" to experiencing chronic pain. It refers to opioid use and/or prescription of opioids in an outpatient setting for chronic pain resulting from a complication of the participant's bleeding disorder. Opioid use for chronic pain is reported as Yes, No, or Unknown. For participants who reported "Yes" to using opioids for chronic pain, the frequency of use is also recorded.
Yes: Participant had used opioids for the treatment of chronic pain related to their bleeding disorder.
No: Participant had not used opioids for the treatment of chronic pain related to his/her bleeding disorder.
Unknown: It was unknown whether participant had used opioids for the treatment of chronic pain related to his/her bleeding disorder.
Other Medical Conditions (graph displays Registry data only)
Some medical conditions are recorded for Registry participants. The categories are not mutually exclusive; therefore, a participant may have more than one medical condition and therefore be counted in more than one category. These non-bleeding disorder medical conditions are recorded for Registry participants to better understand the overall health status of the bleeding disorders population.
- Cancer: Hepatocellular carcinoma (HCC), leukemia, and cancer other than HCC and leukemia.
- Bone conditions: Fracture, osteopenia, and osteoporosis.
- Cardiovascular disease: Acute ischemic stroke, atrial fibrillation, coronary artery disease, congestive heart failure, myocardial infarction, and transient ischemic attack.
- Kidney disease: Chronic kidney disease, kidney stones, and nephrotic syndrome.
- Liver disease or failure: Ascites, cirrhosis, hepatic fibrosis, splenomegaly, and varices (esophageal or gastric).
- Thrombosis: Pulmonary embolism and deep vein thrombosis.
- Other medical conditions: Anxiety, depression, diabetes mellitus, hypertension, iron deficiency anemia, and pseudotumor.
Need Help?
To learn how to navigate and use the tool, watch these tutorials.
If you have technical issues while using the Data Visualization Tool, please contact CCDV_TechSupp@cdc.gov
- Venous thromboembolism (VTE) is an eligible diagnosis in the HTC PP; however, the data visualization is limited to bleeding disorder diagnoses. Information about VTE characteristics in the HTC PP can be found in the bi-annual HTC PP Data Reports.
- Using the Age filter may cause the total counts of people in the Registry to be higher than the people in the HTC PP. This difference is due to what data are displayed between the two datasets. The Registry data are from participants at their time of enrollment, so the age for each participant stays the same over time. The HTC PP data is from each participant's most recent annual visit, so the counts for each age group will change as participants get older. Therefore, if you filter by age, the total count of people in the Registry may be higher than the total count of people in the HTC PP because the Registry ages do not change, whereas the HTC PP ages do change over time.
