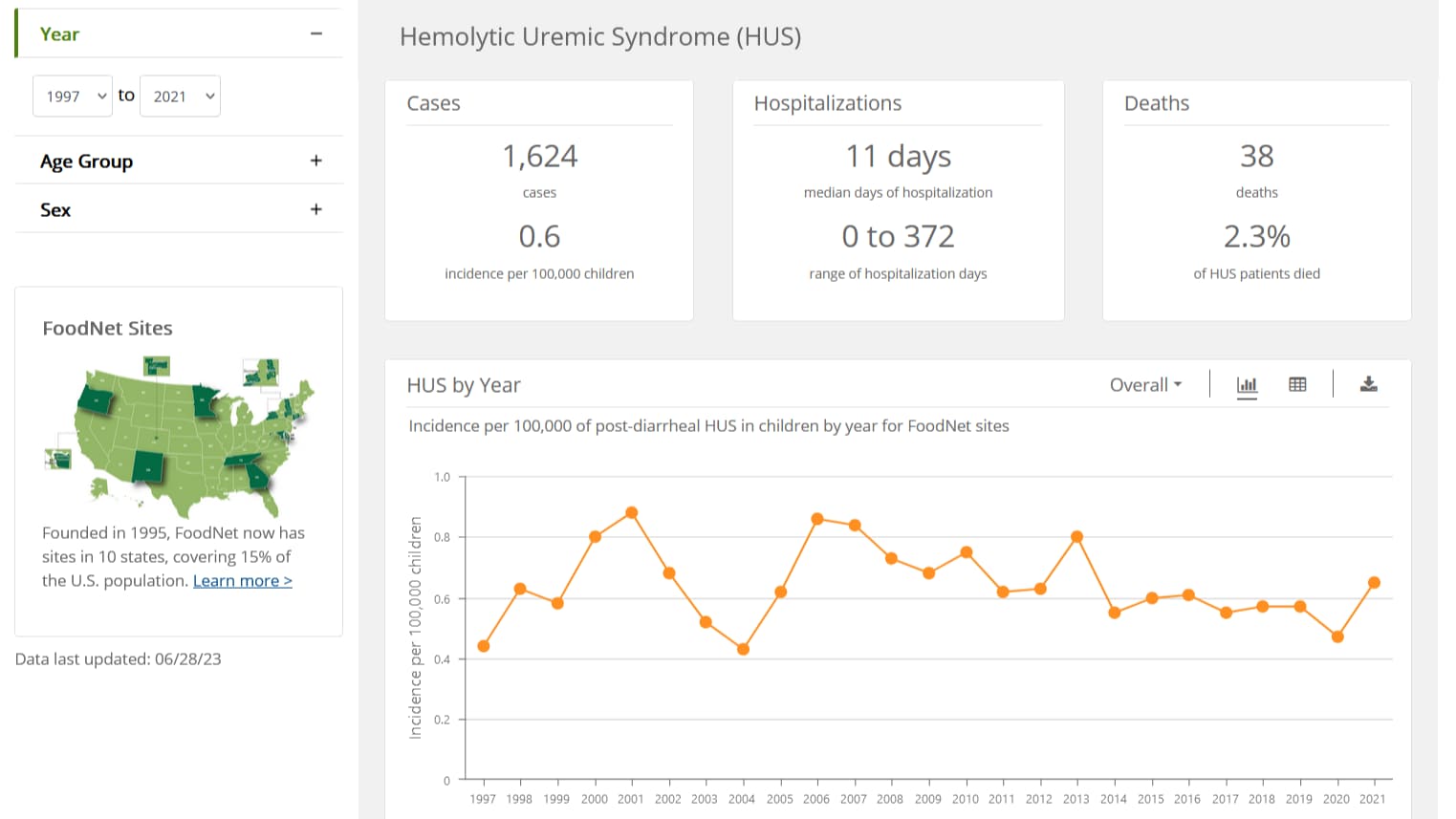
What to know
FoodNet Fast's HUS Surveillance Tool lets you see how rates of HUS have changed in FoodNet's surveillance area since 1997, and how these rates compare with rates of Shiga toxin-producing E. coli (STEC) infection.
Background
Hemolytic Uremic Syndrome (HUS) is a serious condition that can lead to kidney failure, permanent health problems, and even death. HUS is most often triggered by STEC infection, in particular STEC O157. Among children younger than 18 who develop HUS, about 80% have STEC infection.
FoodNet conducts surveillance for physician-diagnosed pediatric post-diarrheal HUS cases.
Information about the data
This tool includes the following information:
- Number of cases, days hospitalized, and deaths
- Incidence per 100,000 children, by year and month
- Demographics, including age and sex
- Evidence of STEC infection
- Comparisons of HUS and STEC incidence
Keep in mind
The data come from a network of pediatric nephrologists and infection control practitioners in FoodNet’s surveillance area and from hospital discharge data review conducted by epidemiologists.
HUS can also be triggered by Streptococcus pneumoniae infection. Other infections have been reported in association with HUS, but whether they caused HUS is not known.
FoodNet tracks the incidence of infections and HUS to assess the effectiveness of measures aimed at preventing infections and to monitor progress toward national health goals.
The CDC laboratory accepts serum specimens sent through state health departments from patients with HUS. These are sent from about 20% of pediatric patients with HUS in FoodNet sites, most often when STEC is not detected in a stool specimen.
A unique test developed and validated at CDC is used for detection of E. coli O157 antibodies in serum. If E. coli O157 antibodies are present at an established threshold, FoodNet considers this a serology-positive E. coli O157 result.
The review of hospital charts in FoodNet’s surveillance area can take one to two years to complete. FoodNet staff at CDC review all HUS data submitted, which also takes time. For these reasons, HUS data typically lag a year behind other FoodNet data releases.
Resources
Start exploring FoodNet Fast now – or click the links below for more information.
