At a glance

Summary
Vaccination of pregnant women with influenza (flu) vaccine, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap), and COVID-19 vaccine can decrease the risk for flu, pertussis (whooping cough), and COVID-19 among pregnant women and their infants. The Advisory Committee on Immunization Practices (ACIP) recommends that all women who are or might be pregnant during the flu season receive flu vaccine, which can be administered at any time during pregnancy1. ACIP also recommends that women receive Tdap vaccine during each pregnancy, preferably during the early part of gestational weeks 27–3623. In addition, vaccination with an updated COVID-19 vaccine is recommended for all people aged ≥ 6 months, including women who are pregnant, breastfeeding, or trying to get pregnant now, or might become pregnant4; updated 2023–2024 COVID-19 vaccines became available in September 20235. To assess coverage with flu, Tdap, and COVID-19 vaccines among women pregnant during the 2023–24 flu season, CDC analyzed data from an Internet panel survey conducted during April 2024. Among 1,783 survey respondents who were pregnant anytime during October 2023–January 2024, 47.4% reported receiving flu vaccine before or during their pregnancy. Among 788 respondents who had a live birth by their survey date, 59.6% reported receiving Tdap vaccine during pregnancy. Among 2,005 women who were pregnant anytime from October 2023 through the time of survey completion, 30.9% reported receipt of an updated 2023–2024 COVID-19 vaccine before or during their pregnancy. For all vaccines, coverage was higher among women who reported receiving a provider offer or referral for vaccination compared with women who did not. Reasons reported for non-vaccination continue to highlight concerns about perceived safety risks if vaccinated during pregnancy, as well as a lack of knowledge regarding the need to receive a Tdap vaccine during every pregnancy67. Provider offers or referrals for vaccination in combination with tailored conversations to educate patients and address their concerns could help increase flu, Tdap, and COVID-19 vaccination coverage among pregnant women.
Methods
An Internet panel survey was conducted to assess end-of-season flu, Tdap, and COVID-19 vaccination coverage among women pregnant during the 2023–24 flu season;A similar surveys have been conducted since April 2011. The survey was conducted during March 26–April 11, 2024, among women aged 18–49 years who reported being pregnant anytime since August 1, 2023, through the date of their survey. Participants were recruited from a large, pre-existing, opt-in Internet panel of the general population operated by Dynata (Dynata_Panel_Book.pdf) through both Dynata's GenPop and Dynamix systems. Among 15,487 women who elected to answer the screening questions, 2,473 were eligible, and of these, 2,266 completed the survey (completion rateB 91.6%). Data were weighted to reflect pregnancy status and outcome at the time of interview, age, race and ethnicity, and geographic distribution of the total U.S. population of pregnant women. Analysis of flu vaccination coverage was limited to 1,783 women pregnant anytime during October 2023–January 2024. A woman was considered to have been vaccinated against flu if she reported having received a dose of flu vaccine since July 1, 2023, and before or during her most recent pregnancy. To accommodate the optimal timing for Tdap vaccination during 27–36 weeks' gestation, analysis of Tdap coverage was limited to women pregnant anytime since August 1, 2023, who had a live birth by their survey date. Among 883 women with a recent live birth, 95 (10.7%) were excluded because they did not know whether they had ever received Tdap (8.8%) or did not know whether they received it during their pregnancy (1.9%), leaving a final analytic sample of 788. A woman was considered to have received Tdap if she reported receiving a dose of Tdap during her most recent pregnancy. Coverage with an updated COVID-19 vaccine (2023–24 formula) was assessed among 2,005 women who were pregnant anytime from October 2023 (after updated 2023–2024 vaccines became available in September 2023) through the time of survey completion and were considered vaccinated if they reported receiving an updated 2023–2024 COVID-19 vaccine before or during pregnancy. Finally, we assessed place of flu, Tdap, and COVID-19 vaccination among vaccinated women, and reasons for not receiving these vaccines among unvaccinated women. SAS-callable SUDAAN software (version 11.0.4; RTI International) was used to conduct all analyses. Differences between groups were determined using t-tests with significance set at p<0.05. Increases or decreases noted in this report represent statistically significant differences.
Results
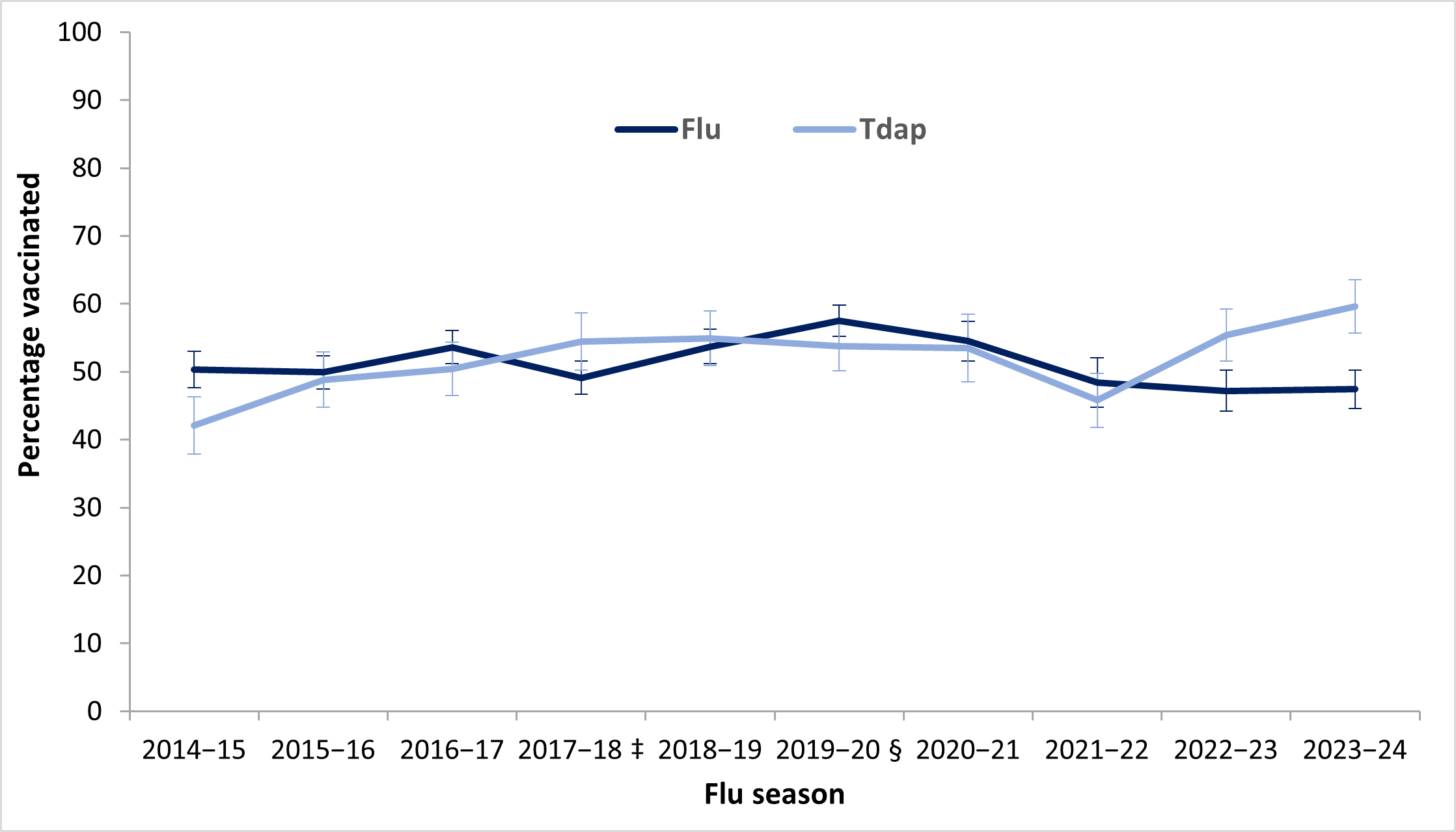
Among women pregnant anytime during October 2023–January 2024, 47.4% reported receiving a dose of flu vaccine since July 1, 2023. (Figure 1, Table). Tdap vaccination coverage during pregnancy was 59.6% among women with a recent live birth. Flu vaccination coverage was similar during the past 3 seasons (48.4%, 47.2%, and 47.4% in the 2021–22, 2022–23, and 2023–24 seasons, respectively). Tdap coverage in 2023–24 (59.6%) was similar to coverage in 2022–23 (55.4%), but has increased significantly since the 2021–22 season (45.8%). Flu and Tdap vaccination coverage estimates for influenza seasons 2014–15 through 2023–24 are reported in a Supplementary Table. Receipt of an updated 2023–2024 COVID-19 vaccine was reported by 30.9% of women who were pregnant anytime from October 2023 through the time of survey completion (Table). Flu vaccination coverage was similar among all race and ethnicity groups studied. Tdap coverage was lowest among non-Hispanic Black (Black) women (47.3%), whereas coverage among Hispanic women (63.0%) was similar to coverage among non-Hispanic White (White) women (61.0%). Conversely, coverage with an updated 2023–2024 COVID-19 vaccine among White women (27.0%) was similar to coverage among Black women (29.0%), and lower than coverage among Hispanic women (38.6%). For all three vaccines, coverage was lower among women with a college degree or less education compared with women who had more than a college degree. Other differences by demographic characteristics are shown in the Table.
For all three vaccines, coverage was highest among women with a provider offer or referral (50.5%–76.1%) and lowest among women with no recommendation (0.3%–16.2%) (Table). Receipt of a provider offer or referral for flu vaccination during the 2023–24 flu season was reported by 72.4% of women pregnant between October and January; 5.2% reported receiving a provider recommendation but no offer or referral, and 22.3% reported not receiving a provider recommendation for flu vaccine. Among women with a live birth, 78.0% reported receipt of an offer or referral for Tdap, while 2.0% received a provider recommendation with no offer or referral, and 20.0% did not receive a recommendation for Tdap. Among women pregnant from October 2023 through the time of survey completion, 51.8% reported receipt of an offer or referral for vaccination with an updated 2023–2024 COVID-19 vaccine, 5.8% received a recommendation but no offer or referral, and 42.4% did not receive a recommendation for an updated 2023–2024 COVID-19 vaccine.
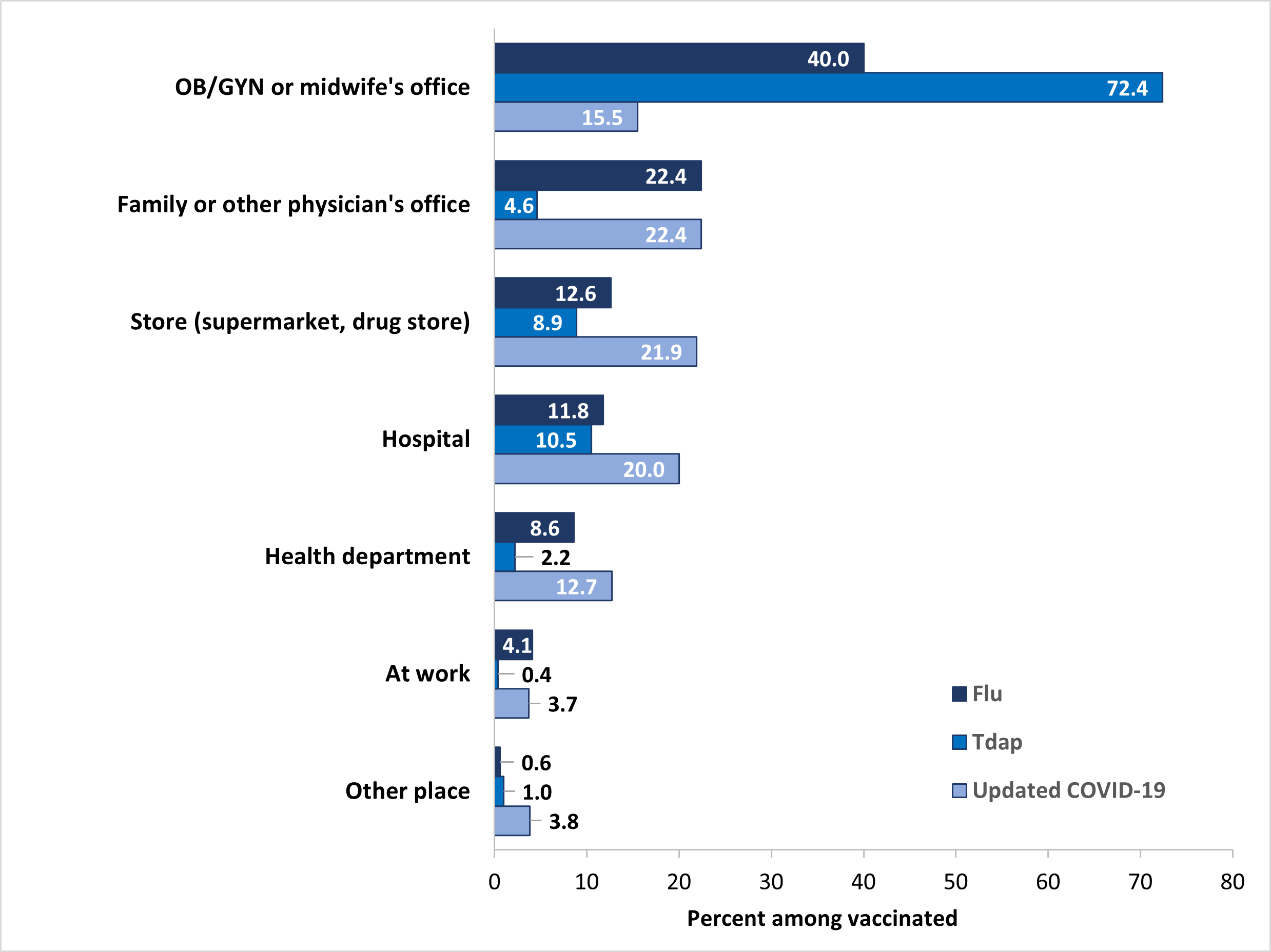
Place of flu vaccination among pregnant women in the 2023–24 flu season was most often the office of an obstetrician/gynecologist (OB/GYN) or midwife (40.0%), followed by a family or other physician's office (22.4%), store (supermarket, drug store, pharmacy) (12.6%), hospital (11.8%), and health department (8.6%) (Figure 2). The most commonly reported place of Tdap vaccination among women with a live birth was an OB/GYN or midwife's office (72.4%), followed by a hospital (10.5%), and store (8.9%). Place of vaccination with an updated 2023–2024 COVID-19 vaccine among pregnant women was most often a family or other physician's office (22.4%), followed by a store (21.9%), hospital (20.0%), an OB/GYN or midwife's office (15.5%), and a health department (12.7%).
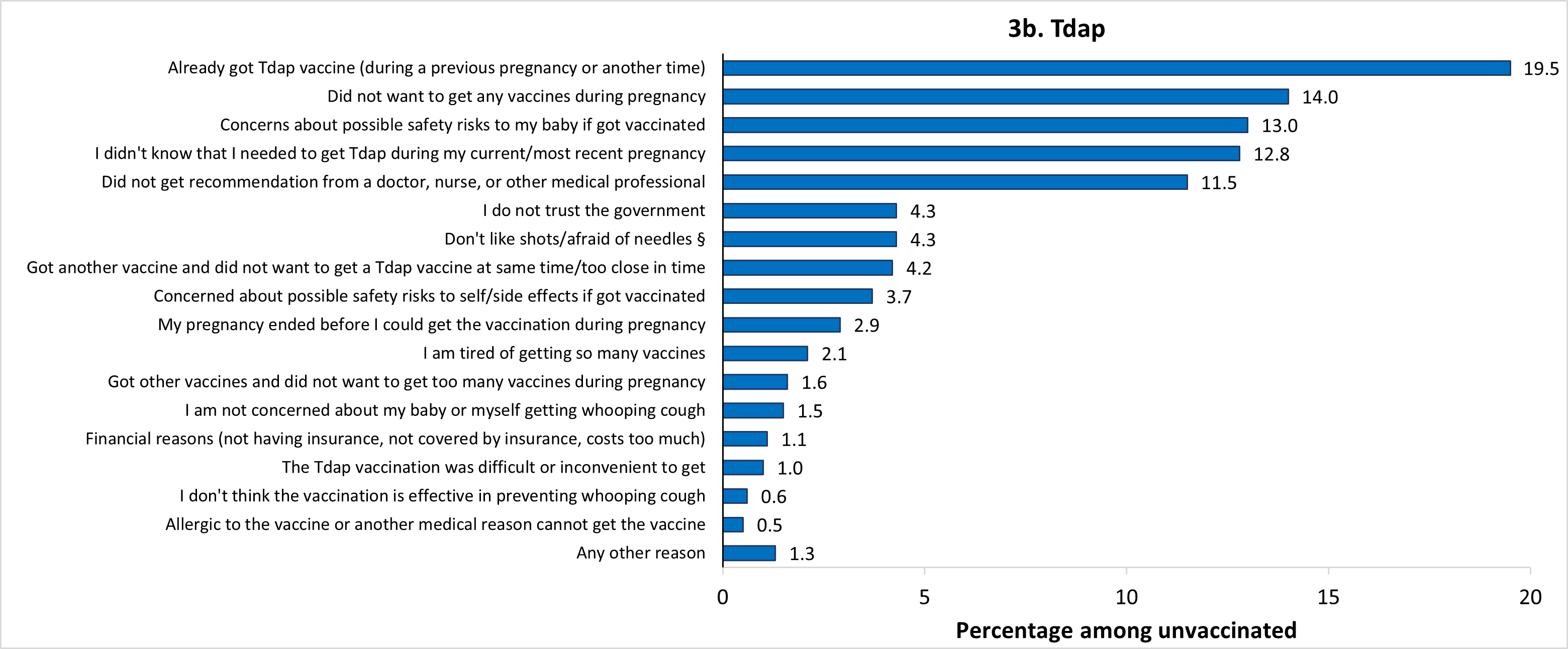
The most frequently reported main reasons for not receiving flu vaccination before or during pregnancy were concern about the safety of their baby if they were to get vaccinated (14.7%), and just not wanting the vaccine (14.0%) (Figure 3a). The most common main reason for not receiving Tdap during pregnancy was that they already got the Tdap vaccine (during a previous pregnancy or at another time) (19.5%) (Figure 3b). The most common main reason for not receiving an updated 2023–2024 COVID-19 vaccine before or during pregnancy was just not wanting the vaccine (16.3%) (Figure 3c). The percent of unvaccinated women who reported not wanting to receive any vaccines during pregnancy as their main reason for not receiving flu, Tdap, and updated 2023–2024 COVID-19 vaccines was 8.9%, 14.0%, and 5.9%, respectively. Other reported main reasons for non-vaccination are shown in Figure 3.
Discussion
Findings from this survey indicate that approximately half of pregnant women did not receive a flu vaccine, nearly 40% did not receive a Tdap vaccine, and about 70% did not receive an updated 2023–2024 COVID-19 vaccine. Flu vaccination coverage has been similar over the past few seasons, and was 10.1 percentage points lower in the 2023–24 season (47.4%) than pre-pandemic coverage in the 2019−20 season (57.5%). Tdap coverage also decreased during the pandemic, from 2019–20 (53.8%) to 2021–22 (45.8%), but then increased 13.8 percentage points from the 2021–22 season (45.8%) to the 2023–24 season (59.6%). Not receiving these recommended vaccines leaves pregnant women and their infants more vulnerable to flu, whooping cough, and COVID-19 and potentially serious complications including adverse birth outcomes, hospitalization, and death89101112.
Similar to findings in previous reports, flu, Tdap, and COVID-19 vaccination coverage was highest among pregnant women with a provider offer or referral for vaccination13. Yet more than one-quarter and one-fifth of women indicated not receiving a provider recommendation for vaccination with flu or Tdap vaccines, respectively, and more than 40% reported not receiving a provider recommendation for vaccination with an updated 2023–2024 COVID-19 vaccine. Providers are encouraged to follow the Standards for Adult Immunization Practice by assessing vaccination status at every clinical encounter, strongly recommending vaccines that their patients need, administering needed vaccines or referring patients to a vaccination provider, and documenting vaccines administered in the immunization information system14. CDC has resources to assist providers in effectively communicating the importance of vaccination, such as sharing specific reasons why recommended vaccines are right for the patient and highlighting positive personal or clinical experiences with vaccines. Another available resource is the ACOG immunization toolkit, which includes communication strategies for providers, as well as extensive information on vaccine financing and coding that could address perceived financial barriers, a commonly reported barrier to stocking vaccines in provider offices1516.
In a previous study of women in the April 2023 Internet Panel Survey, we found that pregnant women commonly reported being hesitant about flu (60%) and Tdap (43%) vaccinations during pregnancy, and that being hesitant was associated with lower vaccination coverage17. The 2023 study also showed that the association between hesitancy and non-vaccination was weaker among women who received a provider recommendation, particularly an offer or referral for vaccination17. In the current study, reasons for non-vaccination highlight maternal concerns about the safety of flu, Tdap, and COVID-19 vaccines during pregnancy, particularly with regard to safety risks to the baby, and lack of knowledge about the recommendation to receive Tdap during every pregnancy; similar findings have been reported previously67. Providers can tailor patient communications and education accordingly to help strengthen their recommendations for vaccination and address patient concerns. ACIP recommendations for maternal flu and Tdap vaccination are well established, and studies have consistently affirmed the safety and effectiveness of maternal vaccination for women, fetuses, and infants, including Tdap vaccination for each pregnancy13181920. Data also support the safety of COVID-19 vaccination during pregnancy21. Providers can share this information with their pregnant patients in an effort to improve vaccine confidence and increase acceptance of recommended vaccines.
No differences in flu or COVID-19 vaccination coverage between White and Black women were observed during the 2023–24 season. However, disparities in Tdap coverage continue to be observed for Black women, who had the lowest coverage in 2023–24, despite an increase in Tdap coverage among Black women compared with the previous season13. In the April 2023 Internet panel survey, we found that Black women were more likely than White women to report being hesitant about flu and Tdap vaccinations during pregnancy13. Factors such as knowledge, attitudes and beliefs about vaccines, including mistrust as a result of past medical racism and experimentation, and structural barriers related to accessing vaccines, such as not having regular access to primary care, have been shown to contribute to lower vaccination rates in Black adults generally222324. Resources with a specific focus on outreach to Black women who are pregnant or may become pregnant are available to healthcare providers on CDC's "From Me, To You" website, including posters, short videos, fact sheets, patient portal reminder language, and other communications tools to encourage vaccination during pregnancy with recommended vaccines.
Estimates of vaccination coverage among pregnant women from the Internet panel survey are higher than those based on electronic health records from the Vaccine Safety Datalink (VSD), a collaboration between CDC and multiple integrated health systems2526. Based on data through April 20, 2024, from the VSD, flu vaccination coverage during the 2023–24 season was 38.1% among pregnant women 18–49 years, compared with 47.4% from our Internet panel survey25. Coverage with an updated 2023–2024 COVID-19 vaccine from the VSD was 13.3% among women who were pregnant during the week ending May 11, 2024, compared with 30.9% from our Internet panel survey26. Differences in coverage estimates are likely due to differences in the samples and assessment methodology2728. However, estimates of coverage with an updated 2023–2024 COVID-19 vaccine, in particular, might be higher than those reported from other data sources if some survey respondents had difficulty distinguishing the most recent COVID vaccine from previous booster and bivalent dosesC. Estimates from the VSD data likely underestimate coverage with an updated 2023–2024 COVID-19 vaccine because they rely on data from electronic health systems and immunization information systems so could miss vaccinations received outside of the respective VSD sites, and did not apply any enrollment criteria so might not have an enrollee's complete vaccination history for the entirety of the respiratory virus season.
Despite longstanding ACIP recommendations, maternal vaccination with flu and Tdap vaccines is suboptimal, and missed opportunities to vaccinate are common. COVID-19 vaccination coverage is suboptimal as well. Findings in this report reinforce the strong association between provider recommendation and offer of or referral for vaccination and maternal vaccination. Providers can help ensure that pregnant women are vaccinated against flu, Tdap, and COVID-19. Vaccination coverage of pregnant women with all recommended vaccines could be increased through implementation of patient education about ACIP vaccination recommendations and safety and benefits of maternal vaccination in combination with evidence-based practices, such as screening patients for recommended vaccinations at every opportunity, reminders to notify providers that their patients need vaccinations, and system-level changes that make vaccinations part of the routine workflow, as well as having a culture of vaccination in the office142930. Racial disparities in vaccination coverage could decrease with consistent provider offers or referrals for vaccination, in combination with multicomponent health system and community-based interventions142931323334.
Limitations
Interpretation of the results in this report should take into account several limitations of the estimates produced from the Internet panel survey. First, the sample may not be representative of all pregnant women in the United States because the survey was conducted among a smaller group of volunteers who were already enrolled in a preexisting, national, opt-in, general-population Internet panel rather than a randomly selected sample of all pregnant women in the United States. Some bias might remain after weighting adjustments, for example, due to the exclusion of women with no Internet access and/or the self-selection processes for entry into the panel and participation in the survey. Estimates might be biased if the selection processes for entry into the Internet panel or a woman's decision to participate in this survey were related to receipt of vaccination. Second, due to small sample size, we were not able to assess vaccination coverage separately among some racial and ethnic groups. Third, all results are based on self-report and not validated by medical record review; therefore, coverage estimates might be subject to recall or social desirability bias and could be over- or underestimates. Fourth, for Tdap, coverage estimates might be subject to uncertainty, given the exclusion of 10.7% of women with unknown Tdap vaccination status. Sensitivity analysis showed that actual Tdap coverage could have ranged from 53.0% to 64.1% in 2024. Finally, formal statistics were used to determine differences in vaccination coverage between groups in this non-probability sample35. Despite these limitations, Internet panel surveys are considered a useful assessment tool for timely evaluation of maternal vaccination coverage among pregnant women.
Tables and Figures
* Received flu vaccination before or during pregnancy from July 1st to mid-April among women pregnant during October through January. Flu vaccination coverage estimates from before the 2023−24 season have been reported previously. https://www.cdc.gov/fluvaxview/coverage-by-season/index.html
† Received Tdap vaccination during pregnancy among women who had a live birth since August 1st of the year before survey completion date. Tdap coverage estimates from before the 2023−24 season have been reported previously. https://www.cdc.gov/adultvaxview/publications-resources/
‡ A methodology change increased the proportion of women who were able to complete the 2018 survey on a smartphone or other handheld device and limits the ability to make comparisons to estimates from previous seasons; however, both flu vaccination and Tdap coverage estimates were similar to those reported from the April 2017 survey. https://www.cdc.gov/mmwr/volumes/67/wr/mm6738a3.htm
§ To further minimize bias, a change in the weighting methodology was employed beginning with the 2020–21 survey. Similar to in previous survey years, sample weights for the 2020–21 survey were created so that the distribution of the weighted sample matched population control totals by region, age group, race/ethnicity and age group by race/ethnicity. Beginning with the 2020–21 survey, the sample weights were constructed to additionally match population control totals by current pregnancy status and outcome at the time of the survey as well as all two-way interactions between region, age group, race/ethnicity and current pregnancy status. The new weighting methodology had a minimal effect for most of the characteristics studied. Estimates included here for the 2019−20 season are re-weighted estimates. https://www.cdc.gov/fluvaxview/coverage-by-season/flu-tdap-pregnant-april-2021.html
| Characteristic | Flu vaccine* | Tdap vaccine† | Updated 2023–2024 COVID-19 vaccine‡ |
|||
|---|---|---|---|---|---|---|
| Total N (weighted %) |
Weighted % Vaccinated (95% CI)§ |
Total N (weighted %) |
Weighted % Vaccinated (95% CI) |
Total N (weighted %) |
Weighted % Vaccinated (95% CI) |
|
| Overall | 1783 (100.0) | 47.4 (44.5–50.4) | 788 (100.0) | 59.6 (55.8–63.4) | 2005 (100.0) | 30.9 (28.6–33.3) |
| Age group (years) | ||||||
| 18–24 | 308 (22.2) | 44.4 (37.3–51.7)|| | 121 (18.8) | 57.6 (47.8–67.0) | 351 (22.5) | 32.9 (27.2–39.0) |
| 25–34 | 824 (57.4) | 45.2 (41.2–49.2)|| | 436 (60.7) | 61.3 (56.1–66.2) | 922 (57.4) | 28.8 (25.7–32.1)|| |
| 35–49 (Ref) | 651 (20.4) | 57.0 (52.2–61.7) | 231 (20.5) | 56.6 (49.4–63.5) | 732 (20.1) | 34.7 (30.7–38.8) |
| Race and ethnicity¶ | ||||||
| White, non-Hispanic (Ref) | 1000 (48.7) | 48.8 (45.0–52.6) | 483 (52.3) | 61.0 (56.2–65.5) | 1111 (48.4) | 27.0 (24.0–30.1) |
| Black, non-Hispanic | 285 (17.5) | 44.0 (35.7–52.5) | 114 (13.5) | 47.3 (37.2–57.7)|| | 318 (17.5) | 29.0 (23.4–35.1) |
| Hispanic | 353 (25.1) | 48.5 (42.3–54.6) | 130 (24.7) | 63.0 (53.4–71.8) | 403 (24.9) | 38.6 (33.4–44.0)|| |
| Other, non-Hispanic | 145 (8.7) | 43.6 (34.0–53.6) | 61 (9.5) | 61.1 (46.0–74.7) | 173 (9.2) | 34.4 (26.2–43.4) |
| Education | ||||||
| High school diploma or less | 467 (29.9) | 37.9 (32.5–43.6)|| | 203 (28.9) | 56.1 (48.3–63.8)|| | 526 (29.9) | 27.3 (22.9–32.0)|| |
| Some college, no degree | 405 (23.6) | 42.2 (36.0–48.7)|| | 191 (23.6) | 58.8 (50.9–66.4)|| | 454 (23.6) | 20.9 (16.5–25.9)|| |
| College degree | 657 (35.0) | 52.4 (47.6–57.2)|| | 290 (35.8) | 56.7 (50.2–63.0)|| | 744 (35.1) | 34.9 (31.0–39.0)|| |
| Greater than college degree (Ref) | 254 (11.5) | 67.5 (60.1–74.2) | 104 (11.6) | 78.8 (69.3–86.5) | 281 (11.3) | 48.8 (41.9–55.6) |
| Employment status | ||||||
| Working (Ref) | 1190 (66.3) | 51.2 (47.6–54.9) | 497 (61.4) | 59.0 (54.2–63.7) | 1351 (67.3) | 34.5 (31.5–37.5) |
| Not working | 593 (33.7) | 39.9 (35.1–44.9)|| | 291 (38.6) | 60.6 (54.1–66.8) | 654 (32.7) | 23.5 (19.8–27.6)|| |
| Poverty status** | ||||||
| At or above poverty level (Ref) | 1364 (76.1) | 49.9 (46.6–53.3) | 604 (75.1) | 61.9 (57.6–66.2) | 1543 (76.4) | 33.1 (30.4–35.9) |
| Below poverty level | 418 (23.9) | 39.3 (33.5–45.3)|| | 184 (24.9) | 52.6 (44.4–60.7)|| | 460 (23.6) | 23.4 (19.0–28.3)|| |
| Area of residence†† | ||||||
| Rural | 322 (17.4) | 40.5 (33.8–47.6)|| | 162 (19.7) | 55.7 (47.0–64.2) | 374 (18.0) | 21.1 (16.2–26.7)|| |
| Nonrural (Ref) | 1461 (82.6) | 48.9 (45.7–52.1) | 626 (80.3) | 60.6 (56.3–64.8) | 1631 (82.0) | 33.0 (30.5–35.7) |
| Region‡‡ | ||||||
| Northeast (Ref) | 306 (17.7) | 56.1 (48.9–63.0) | 106 (16.2) | 64.9 (54.5–74.4) | 344 (17.2) | 36.8 (30.8–43.2) |
| Midwest | 404 (20.0) | 47.7 (41.2–54.4) | 194 (20.1) | 62.8 (55.2–69.9) | 449 (19.8) | 28.3 (23.4–33.6)|| |
| South | 750 (38.0) | 46.3 (41.9–50.6)|| | 347 (40.8) | 55.0 (49.1–60.8) | 847 (38.8) | 29.8 (26.4–33.4)|| |
| West | 323 (24.3) | 42.7 (36.1–49.5)|| | 141 (22.9) | 61.3 (52.0–70.1) | 365 (24.2) | 30.6 (25.2–36.4) |
| Prenatal insurance coverage§§ | ||||||
| Private/military insurance only (Ref) | 852 (43.9) | 55.5 (51.3–59.6) | 402 (48.0) | 65.9 (60.6–71.0) | 956 (43.7) | 33.1 (29.7–36.6) |
| Any public insurance | 857 (51.3) | 41.4 (37.1–45.7)|| | 363 (48.6) | 53.5 (47.7–59.2)|| | 965 (51.5) | 28.9 (25.5–32.4) |
| No insurance | 74 (4.8) | 38.1 (25.8–51.7)|| | 23 (3.3) | —|||| | 84 (4.7) | 32.5 (21.5–45.2) |
| Provider vaccination recommendation or offer¶¶ | ||||||
| Offered or referred (Ref) | 1319 (72.4) | 59.6 (56.0–63.0) | 604 (78.0) | 76.1 (72.1–79.7) | 1084 (51.8) | 50.5 (46.8–54.2) |
| Recommended, no offer or referral | 94 (5.2) | 22.1 (13.4–33.1)|| | 17 (2.0) | —|||| | 122 (5.8) | 22.8 (15.0–32.3)|| |
| No recommendation | 352 (22.3) | 16.2 (11.0–22.7)|| | 166 (20.0) | 0.3 (0.0–2.7)|| | 797 (42.4) | 8.2 (6.2–10.6)|| |
| Number of provider visits since July 1, 2023 | ||||||
| None | 15 (1.2) | —|||| | N/A | N/A | N/A | N/A |
| 1–5 | 612 (37.9) | 44.0 (38.8–49.3) | N/A | N/A | N/A | N/A |
| 6–10 | 674 (36.4) | 51.9 (47.1–56.7) | N/A | N/A | N/A | N/A |
| >10 (Ref) | 482 (24.5) | 48.0 (42.9–53.2) | N/A | N/A | N/A | N/A |
| High-risk condition for influenza*** | ||||||
| Yes (Ref) | 848 (50.4) | 49.3 (44.9–53.6) | N/A | N/A | N/A | N/A |
| No | 861 (49.6) | 45.9 (41.8–50.1) | N/A | N/A | N/A | N/A |
Abbreviations: CI: confidence interval; Flu: influenza; N/A: not applicable; Ref: referent group; Tdap: tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine.
* Respondents pregnant anytime during October 2023–January 2024 were included in the analyses to assess flu vaccination coverage for the 2023–24 season. Women who reported receiving a flu vaccination since July 1, 2023, before or during their pregnancy, were considered vaccinated.
† Respondents pregnant since August 1, 2023, and had recently delivered a live birth were included in the analyses to assess Tdap vaccination coverage. Women who reported receiving a Tdap vaccination during their pregnancy were considered vaccinated.
‡ Respondents pregnant anytime from October 2023 through the time of survey completion were included in the analysis. Those who reported receiving an updated 2023–2024 COVID-19 vaccine before or during their pregnancy were considered vaccinated.
§ Korn-Graubard 95% confidence interval.
|| Statistically significant difference compared with referent group.
¶ Race and ethnicity was self-reported. Respondents identified as Hispanic might be of any race. The “Other” race category included Asians, American Indians/Alaska Natives, Native Hawaiians or other Pacific Islanders, and women who selected multiple races.
** Poverty status was defined based on the reported number of persons living in the household and annual household income, according to U.S. Census poverty thresholds. https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-poverty-thresholds.html
†† Rurality was defined using ZIP codes where >50% of the population resides in a nonmetropolitan county, a rural U.S. Census tract, or both, according to the Health Resources and Services Administration’s definition of rural population. https://www.hrsa.gov/rural-health/about-us/definition/index.html
‡‡ Northeast: Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont. Midwest: Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin. South: Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia. West: Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming.
§§ Respondents pregnant on their survey date were asked what medical insurance or medical care coverage they had; respondents who had already delivered were asked what they had during their most recent pregnancy. Women considered to have public insurance selected at least one of the following: Medicaid, Medicare, state-sponsored medical plan, or other government plan. Respondents considered to have private/military insurance selected private medical insurance and/or military medical care and did not select any type of public insurance.
|||| Estimates do not meet the NCHS standards of reliability. https://www.cdc.gov/nchs/data/series/sr_02/sr02_175.pdf
¶¶ Excluded women from flu vaccination analyses who did not report having a provider visit since July 2023 (n=15).
*** Conditions other than pregnancy associated with increased risk for serious medical complications of flu include chronic asthma, a lung condition other than asthma, a heart condition, diabetes, a kidney condition, a liver condition, obesity, or a weakened immune system caused by a chronic illness or by medicines taken for a chronic illness. Women who were missing information were excluded from analysis (n=74).
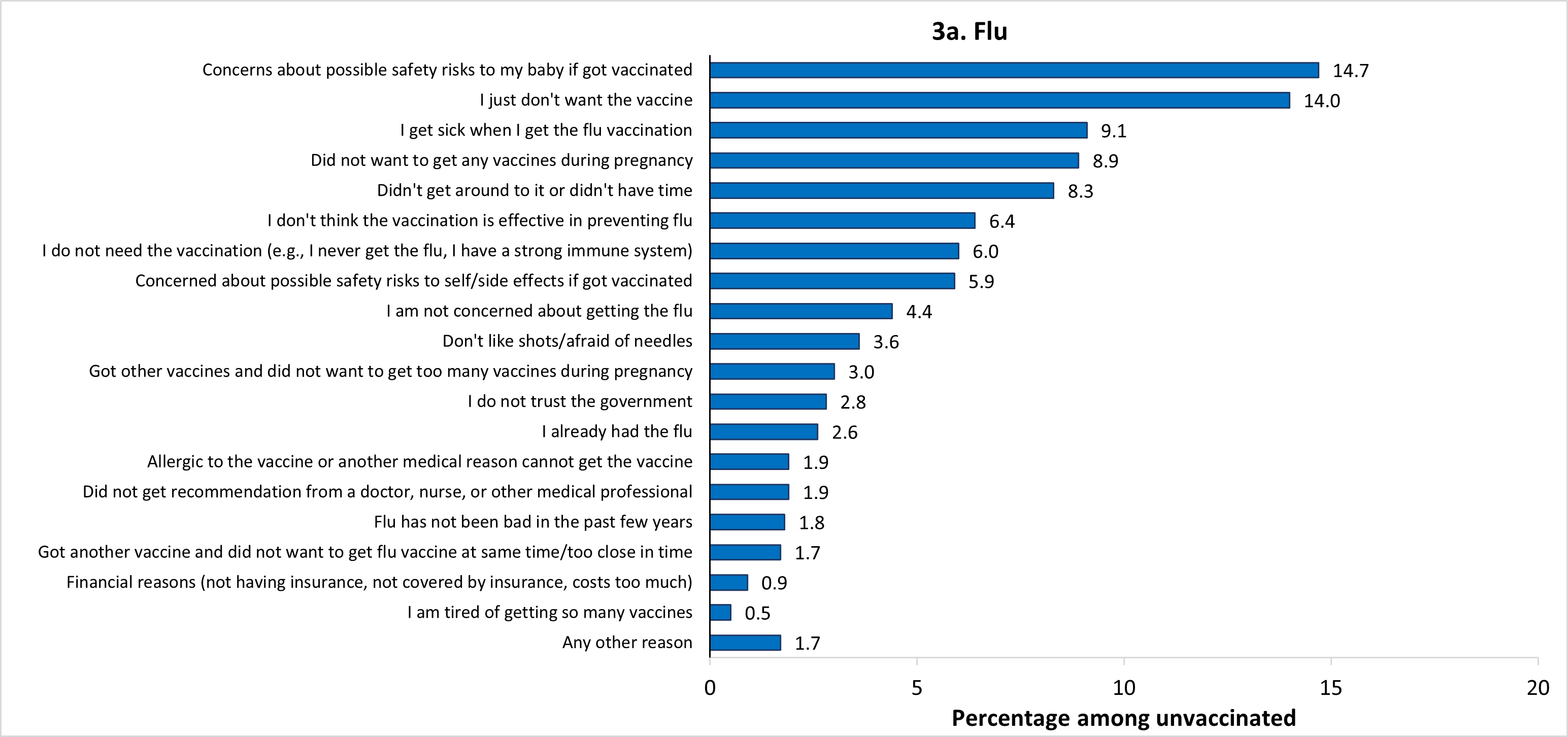
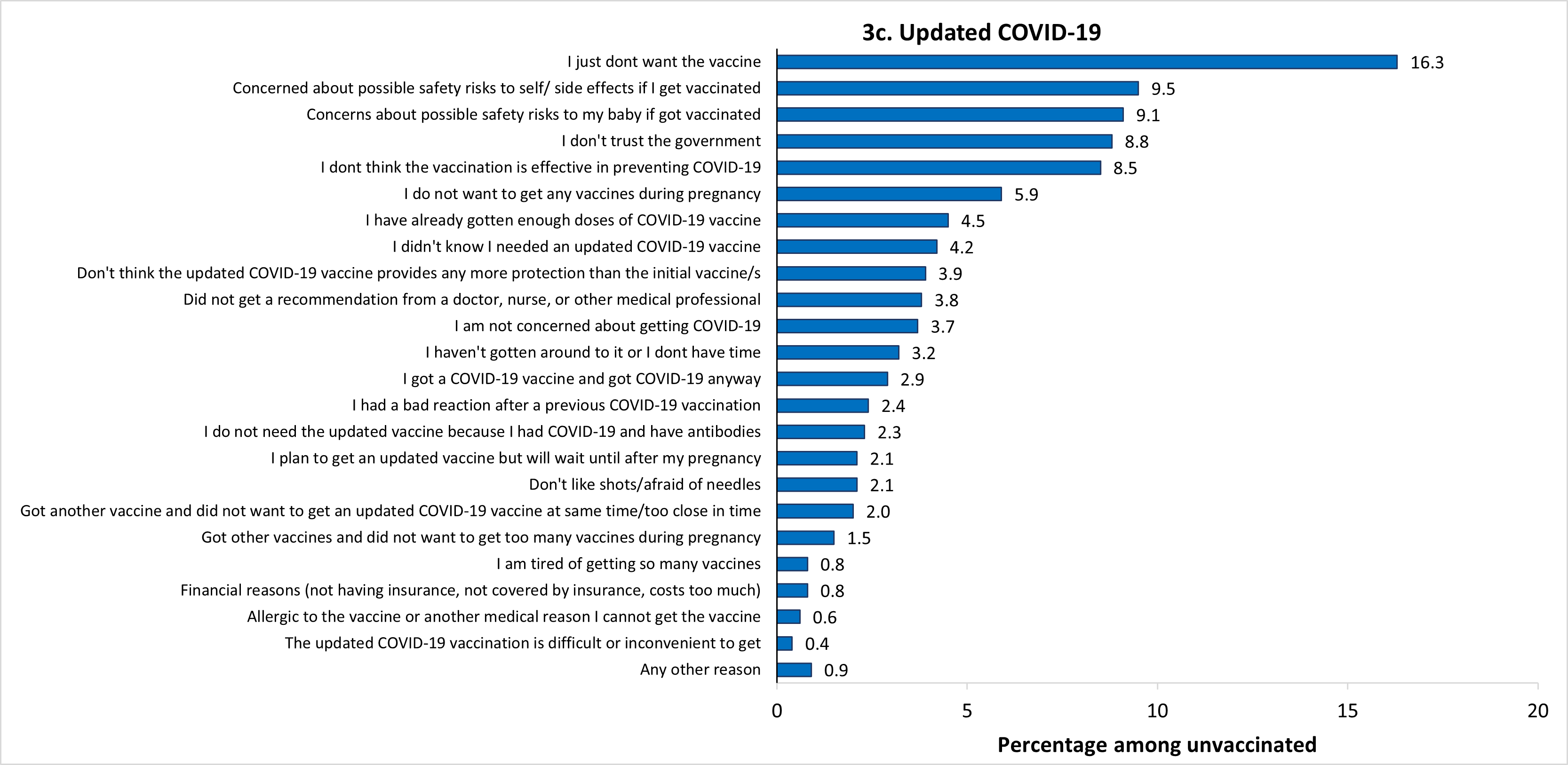
* Among women pregnant during October 2023–January 2024 who reported not receiving a flu vaccination before or during pregnancy (n=856), 1 respondent did not answer the question about reasons for non-vaccination. In addition, 53 respondents reported being vaccinated after pregnancy (not considered vaccinated in the study) and were not asked the reasons question. Therefore, a total of 54 respondents were excluded from the analysis, leaving an analytic sample of 802 women.
† Among women with a live birth since August 1, 2023, who reported not receiving a Tdap vaccination during pregnancy (n=326), 3 respondents reported receiving Tdap before their pregnancy and 1 respondent reported receiving Tdap after their pregnancy (not considered vaccinated in the study) and were not asked the question about reasons for non-vaccination and were excluded from analysis, leaving an analytic sample of 322.
‡ Among women pregnant during October 2023 through the time of survey completion who reported not receiving an updated 2023–2024 COVID-19 vaccine before or during pregnancy (n=1,347), 2 respondents did not answer the question about reasons for non-vaccination. In addition, 29 respondents reported being vaccinated after pregnancy (not considered vaccinated in the study) and were not asked the reasons question. Therefore, a total of 31 respondents were excluded from the analysis, leaving an analytic sample of 1,316 women.
§ Estimates do not meet the NCHS standards of reliability. https://www.cdc.gov/nchs/data/series/sr_02/sr02_175.pdf
| Weighted % vaccinated (95% CI)‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2014−15 | 2015−16 | 2016−17 | 2017−18§ | 2018−19 | 2019−20|| | 2020−21 | 2021−22 | 2022−23 | 2023−24 | |
| Flu | 50.3 (± 2.4) | 49.9 (± 2.4) | 53.6 (± 2.5) | 49.1 (± 2.5) | 53.7 (± 2.3) | 57.5 (± 2.9) | 54.5 (± 3.6) | 48.4 (± 3.1) | 47.2 (± 2.9) | 47.4 (± 2.9) |
| Tdap | 42.1 (± 4.1) | 48.8 (± 3.9) | 50.4 (± 4.2) | 54.4 (± 4.0) | 54.9 (± 3.7) | 53.8 (± 5.0) | 53.5 (± 4.0) | 45.8 (± 3.8) | 55.4 (± 3.9) | 59.6 (± 3.7) |
* Received flu vaccination before or during pregnancy from July 1st to mid-April among women pregnant during October through January. Flu vaccination coverage estimates from before the 2023−24 season have been reported previously. https://www.cdc.gov/fluvaxview/coverage-by-season/index.html
† Received Tdap vaccination during pregnancy among women who had a live birth since August 1st of the year before survey completion date. Tdap coverage estimates from before the 2023−24 season have been reported previously. https://www.cdc.gov/adultvaxview/publications-resources/
‡ Confidence interval half-widths.
§ A methodology change increased the proportion of women who were able to complete the 2018 survey on a smartphone or other handheld device and limits the ability to make comparisons to estimates from previous seasons; however, both flu vaccination and Tdap coverage estimates were similar to those reported from the April 2017 survey. https://www.cdc.gov/mmwr/volumes/67/wr/mm6738a3.htm
|| To further minimize bias, a change in the weighting methodology was employed beginning with the 2020–21 survey. Similar to in previous survey years, sample weights for the 2020–21 survey were created so that the distribution of the weighted sample matched population control totals by region, age group, race/ethnicity and age group by race/ethnicity. Beginning with the 2020–21 survey, the sample weights were constructed to additionally match population control totals by current pregnancy status at the time of the survey as well as all two-way interactions between region, age group, race/ethnicity and current pregnancy status. The new weighting methodology had a minimal effect for most of the characteristics studied. Estimates for 2019−20 included here are re-weighted estimates. https://www.cdc.gov/fluvaxview/coverage-by-season/flu-tdap-pregnant-april-2021.html
Authors
Katherine E. Kahn, MPH1,2; Emma Garacci, MS2,3; Hilda Razzaghi, PhD2; Tara C. Jatlaoui, MD2; Tami H. Skoff, MS4; Sascha R. Ellington, PhD5; Carla L. Black, PhD2
1Eagle Health Analytics, LLC, Atlanta, GA; 2Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; 3Cherokee Nation Operational Solutions, Tulsa, OK; 4Division of Bacterial Disease, National Center for Immunization and Respiratory Diseases, CDC; 5 Influenza Division, National Center for Immunization and Respiratory Diseases, CDC
- Maternal respiratory syncytial virus (RSV) vaccination was also assessed by our 2024 Internet panel survey and a separate report can be found in the Morbidity and Mortality Weekly Report on September 26, 2024. https://www.cdc.gov/mmwr/volumes/73/wr/mm7338a2.htm
- A survey response rate requires specification of the denominator at each stage of sampling. During recruitment of an online opt-in survey sample, such as the Internet panels described in this report, these numbers are not available; therefore, a response rate cannot be calculated. Instead, the survey completion rate is provided.
- Respondents were asked, "An updated COVID-19 vaccine (2023–2024 formula) became available in September 2023. Have you ever received an updated COVID-19 vaccine?"
- Grohskopf, L.A., et al., Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2024-25 influenza season. MMWR Recomm Rep, 2024. 73(5): p. 1-25. https://www.cdc.gov/mmwr/volumes/73/rr/rr7305a1.htm
- Havers, F.P., et al., Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: Updated recommendations of the Advisory Committee on Immunization Practices - United States, 2019. MMWR Morb Mortal Wkly Rep, 2020. 69(3): p. 77-83. https://www.cdc.gov/mmwr/volumes/69/wr/mm6903a5.htm
- Liang, J.L., et al., Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep, 2018. 67(2): p. 1-44. https://www.cdc.gov/mmwr/volumes/67/rr/rr6702a1.htm
- CDC. COVID-19 vaccination for women who are pregnant or breastfeeding. 2024 [cited 08/07/2024]. Available from: https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html
- Regan, J.J., et al., Use of updated COVID-19 vaccines 2023-2024 formula for persons aged ≥6 months: Recommendations of the Advisory Committee on Immunization Practices - United States, September 2023. MMWR Morb Mortal Wkly Rep, 2023. 72(42): p. 1140-1146. https://www.cdc.gov/mmwr/volumes/72/wr/mm7242e1.htm
- Kahn, K.E., et al. Flu and Tdap vaccination coverage among pregnant women – United States, April 2021. 2021 [cited 08/27/2024]; Available from: https://www.cdc.gov/fluvaxview/coverage-by-season/flu-tdap-pregnant-april-2021.html
- Razzaghi, H., et al., COVID-19 vaccination and intent among pregnant women, United States, April 2021. Public Health Rep, 2022. 137(5): p. 988-999.
- Allotey, J., et al., Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. Bmj, 2020. 370: p. m3320.
- Lindley, M.C., et al., Vital Signs: Burden and prevention of influenza and pertussis among pregnant women and infants - United States. MMWR Morb Mortal Wkly Rep, 2019. 68(40): p. 885-892. https://www.cdc.gov/mmwr/volumes/68/wr/mm6840e1.htm
- Wei, S.Q., et al., The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. Cmaj, 2021. 193(16): p. E540-e548.
- Woodworth, K.R., et al., Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep, 2020. 69(44): p. 1635-1640. https://www.cdc.gov/mmwr/volumes/69/wr/mm6944e2.htm
- Zambrano, L.D., et al., Update: Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep, 2020. 69(44): p. 1641-1647. https://www.cdc.gov/mmwr/volumes/69/wr/mm6944e3.htm
- Razzaghi, H., et al., Influenza, Tdap, and COVID-19 vaccination coverage and hesitancy among pregnant women - United States, April 2023. MMWR Morb Mortal Wkly Rep, 2023. 72(39): p. 1065-1071. https://www.cdc.gov/mmwr/volumes/72/wr/mm7239a4.htm
- Orenstein, W.A., et al., Recommendations from the National Vaccine Advisory committee: Standards for adult immunization practice. Public Health Rep, 2014. 129(2): p. 115-23.
- ACOG. Immunization for women, physician tools. 2024 [cited 08/27/2024]; Available from: https://www.acog.org/programs/immunization-for-women/physician-tools?utm_source=redirect&utm_medium=web&utm_campaign=int
- O'Leary, S.T., et al., Immunization practices of U.S. obstetrician/gynecologists for pregnant patients. Am J Prev Med, 2018. 54(2): p. 205-213.
- Calhoun, K., et al. Association of vaccine hesitancy with maternal influenza and Tdap vaccination coverage – United States, 2019–20 to 2022–23. 2023 [cited 08/07/2024]; Available from: https://www.cdc.gov/fluvaxview/coverage-by-season/pregnant-2022-2023.html
- Skoff, T.H., et al., Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants <2 months of age: A case-control evaluation. Clin Infect Dis, 2017. 65(12): p. 1977-1983.
- Skoff, T.H., et al., US infant pertussis incidence trends before and after implementation of the maternal tetanus, diphtheria, and pertussis vaccine. JAMA Pediatr, 2023. 177(4): p. 395-400.
- Sukumaran, L., et al., Association of Tdap vaccination with acute events and adverse birth outcomes among pregnant women with prior tetanus-containing immunizations. Jama, 2015. 314(15): p. 1581-7.
- Prasad, S., et al., Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun, 2022. 13(1): p. 2414.
- Brewer, L.I., et al., Structural inequities in seasonal influenza vaccination rates. BMC Public Health, 2021. 21(1): p. 1166.
- Lu, P.J., et al., Trends in racial/ethnic disparities in influenza vaccination coverage among adults during the 2007-08 through 2011-12 seasons. Am J Infect Control, 2014. 42(7): p. 763-9.
- Uscher-Pines, L., J. Maurer, and K.M. Harris, Racial and ethnic disparities in uptake and location of vaccination for 2009-H1N1 and seasonal influenza. American Journal of Public Health, 2011. 101(7): p. 1252-1255.
- CDC. Influenza vaccination coverage, pregnant women, United States. 2024 [cited 08/07/2024]; Available from: https://www.cdc.gov/fluvaxview/dashboard/pregnant-women-coverage.html
- CDC. COVID-19 vaccination coverage, pregnant women, United States. 2024 [cited 08/07/2024]; Available from: https://www.cdc.gov/covidvaxview/weekly-dashboard/pregnant-women-vaccination.html
- Naleway, A.L., et al., Vaccine Safety Datalink infrastructure enhancements for evaluating the safety of maternal vaccination. Ther Adv Drug Saf, 2021. 12: 20420986211021233.
- Razzaghi, H., et al., COVID-19 vaccination coverage among pregnant women during pregnancy - Eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep, 2021. 70(24): p. 895-899. https://www.cdc.gov/mmwr/volumes/70/wr/mm7024e2.htm
- Community Preventive Services Task Force, The Community Guide. 2016 [cited 08/27/2024]; Available from: https://www.thecommunityguide.org/topic/vaccination
- Mazzoni, S.E., et al., Effect of a multi-modal intervention on immunization rates in obstetrics and gynecology clinics. Am J Obstet Gynecol, 2016. 214(5): p. 617.e1-7.
- Gagneur, A., et al., From vaccine hesitancy to vaccine motivation: A motivational interviewing based approach to vaccine counselling. Hum Vaccin Immunother, 2024. 20(1): p. 2391625.
- Galea, S., S. Sisco, and D. Vlahov, Reducing disparities in vaccination rates between different racial/ethnic and socioeconomic groups: the potential of community-based multilevel interventions. J Ambul Care Manage, 2005. 28(1): p. 49-59.
- Jarrett, C., et al., Strategies for addressing vaccine hesitancy – A systematic review. Vaccine, 2015. 33(34): p. 4180-4190.
- Liu, S., et al., A systematic review and meta-analysis of strategies to promote vaccination uptake. Nat Hum Behav, 2024.
- Baker, R., et al. Report of the AAPOR task force on non-probability sampling. 2013 [cited 08/27/2024]; Available from: https://aapor.org/wp-content/uploads/2022/11/NPS_TF_Report_Final_7_revised_FNL_6_22_13-2.pdf
