What to know
Established in 2019, the Virtual SARS-CoV-2, Influenza, and Other respiratory viruses Network (VISION) is a research collaboration between CDC, Westat, and multiple health systems in the United States with integrated medical, laboratory, pharmacy, billing, and vaccination records and linkages to state and local immunization information systems. The network evaluates how well vaccines and preventive products (e.g., monoclonal antibodies) work to protect people from illness and complications caused by infection with SARS-CoV-2, seasonal influenza viruses, and respiratory syncytial virus (RSV).
Background
VISION's primary objective is to assess COVID-19, influenza (flu), and RSV vaccine and preventive product effectiveness against moderate and severe outcomes and across age groups, care settings, and population subgroups (e.g., people with immunocompromising conditions). These data help inform how well COVID-19, flu, and RSV vaccines and preventive products protect against moderate and severe outcomes of these illnesses.
Secondary objectives include:
1) Assessing COVID-19, flu, RSV, and human metapneumovirus (hMPV) testing frequency, test type, and test results across age groups, care settings, disease severity, and population subgroups.
2) Assessing uptake of COVID-19, flu, and RSV vaccines and preventive products by dose number and product type among people hospitalized with acute respiratory infection.
3) Describing the epidemiology and clinical characteristics of COVID-19, flu, RSV, and hMPV across age groups, care settings, moderate and severe outcomes, and population subgroups.
4) Describing the trajectory of care for individuals with COVID-19, flu, RSV, and hMPV, including severe outcomes (e.g., ICU admission, mechanical ventilation, in-hospital death) and post-acute sequelae (e.g., new-onset clinical diagnoses).
5) Assessing use of therapies, including antiviral drugs, for the treatment and prophylaxis of respiratory viruses and evaluating the ability of these therapies to prevent illness and/or reduce disease severity.
Sites and study design
VISION is composed of eight participating sites in nine different states:
- Kaiser Permanente Northern California (California)
- Kaiser Permanente Southern California (California)
- University of Colorado (Colorado)
- Regenstreif Institute (Indiana)
- HealthPartners Institute (Minnesota; Wisconsin)
- Columbia University (New York)
- Intermountain Health (Utah)
- Kaiser Permanente Center for Health Research (Washington; Oregon)
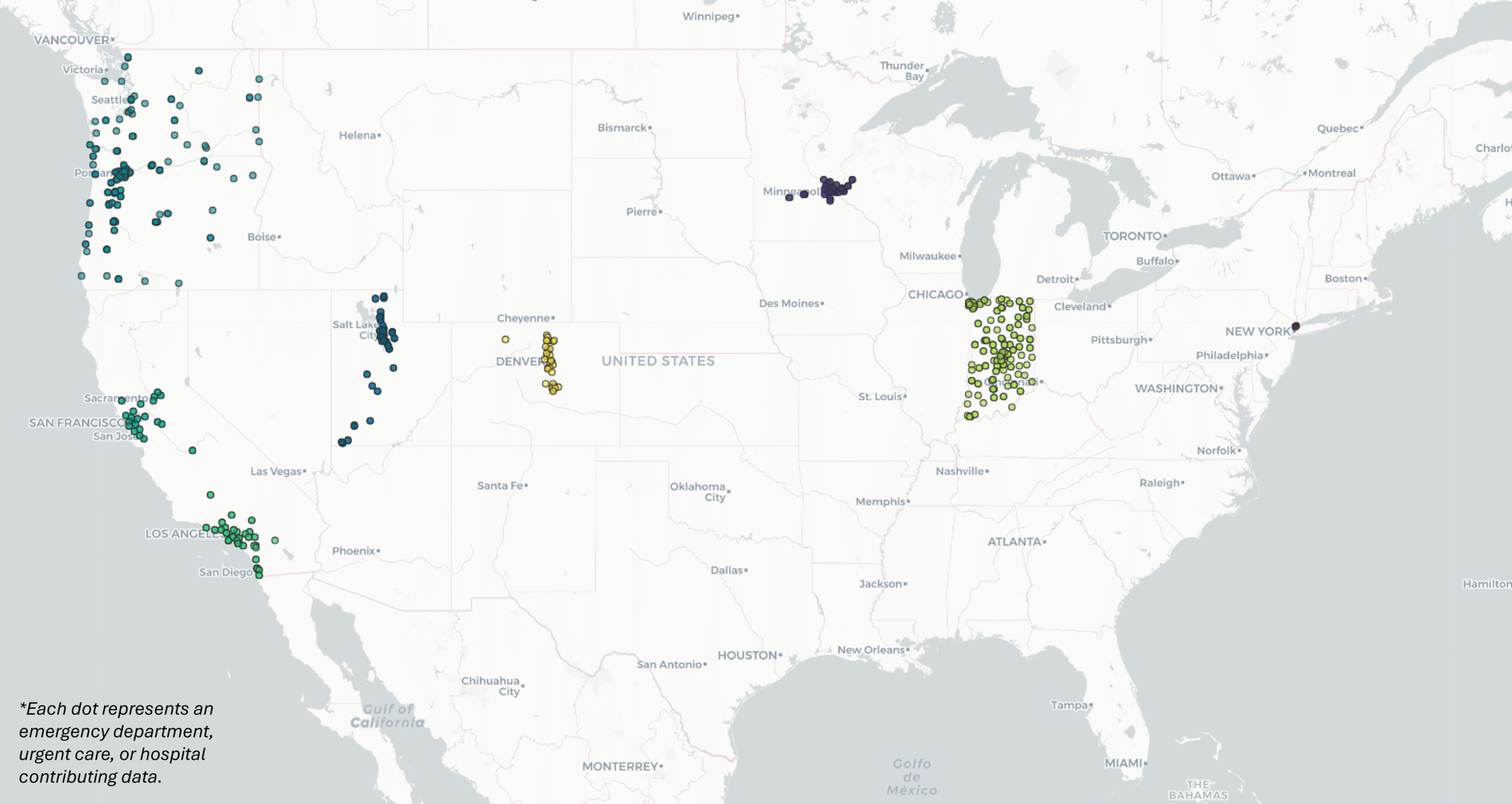
VISION uses a test-negative case-control design as well as a prospective cohort design with data collected by participating sites.
VISION publications
2025
- Frutos AM, Cleary S, Reeves EL, et al. Interim Estimates of 2024–2025 Seasonal Influenza Vaccine Effectiveness — Four Vaccine Effectiveness Networks, United States, October 2024–February 2025. MMWR Morb Mortal Wkly Rep 2025;74:83–90. DOI: http://dx.doi.org/10.15585/mmwr.mm7406a2.
- Influenza Vaccine Effectiveness Against Hospitalizations and Emergency Department or Urgent Care Encounters for Children, Adolescents, and Adults During the 2023-2024 Season, United States.Tenforde MW, Reeves EL, Weber ZA, Tartof SY, Klein NP, Dascomb K, DeSilva MB, Yang DH, Grannis SJ, Irving SA, Ong TC, Link-Gelles R, Salas SB, Sy LS, Lewin B, Contreras R, Zerbo O, Fireman B, Hansen J, Timbol J, Sheffield T, Bride D, Arndorfer J, VanOtterloo J, McEvoy CE, Akinsete OO, Essien IJ, Dixon BE, Rogerson C, Fadel WF, Duszynski T, Naleway AL, Barron MA, Rao S, Mayer D, Chavez C, Ball SW, Payne AB, Ray C, Dickerson M, Neelam V, Adams K, Flannery B, DeCuir J, Garg S.Clin Infect Dis. 2025 Oct 6;81(3):667-678. doi: 10.1093/cid/ciae597.
- Effectiveness of nirsevimab among infants in their first RSV season in the United States, October 2023-March 2024: a test-negative design analysis.Payne AB, Battan-Wraith S, Rowley EAK, Stockwell MS, Tartof SY, Dascomb K, Irving SA, Dixon B, Ball SW, Tenforde MW, Vazquez-Benitez G, Stephens AB, Han J, Natarajan K, Salas SB, Bezi C, Sy LS, Lewin B, Sheffield T, Arndorfer J, Bride D, Van Otterloo J, Naleway AL, Koppolu PD, Grannis S, Fadel W, Rogerson C, Duszynski T, Reese SE, Mitchell PK, Chickery S, Moline HL, Najdowski M, Ciesla AA, Reeves EL, DeSilva M, Fleming-Dutra KE, Link-Gelles R.
- Effectiveness of 2023-2024 seasonal influenza vaccine against influenza-associated emergency department and urgent care encounters among pregnant and non-pregnant women of reproductive age.Reeves EL, Dascomb K, Irving SA, Klein NP, Tartof SY, Grannis SJ, Ong TC, Ball SW, Vazquez-Benitez G, Sheffield T, Bride D, Arndorfer J, Van Otterloo J, Naleway AL, Koppolu P, Zerbo O, Jacobson KB, Fireman BH, Hansen JR, Block L, Salas SB, Bezi C, Sy LS, Reyes IAC, Dixon BE, Fadel WF, Rogerson C, Duszynski T, Mayer D, Chavez C, Barron MA, Weber ZA, Yang DH, Cheung A, Payne AB, Link-Gelles R, Adams K, Neelam V, DeSilva MB, Natarajan K, Tenforde MW, DeCuir J, Ellington S, Olson SM.Vaccine. 2025 Aug 30;62:127483. doi: 10.1016/j.vaccine.2025.127483. Epub 2025 Jul 13.PMID: 40660660
- Estimated 2023-2024 COVID-19 Vaccine Effectiveness in Adults.Link-Gelles R, Rowley EAK, Irving SA, Klein NP, Grannis SJ, Ong TC, Ball SW, DeSilva MB, Dascomb K, Naleway AL, Koppolu P, Zerbo O, Fireman B, Hansen J, Timbol J, Block L, Dixon BE, Duszynski TJ, Allen KS, Mayer D, Chavez C, Barron M, Reese SE, Chickery S, Davis JM, Ciesla AA, Mak J, Najdowski M, Akinsete OO, McEvoy CE, Essien IJ, Sheffield T, Bride D, Arndorfer J, Van Otterloo J, Natarajan K, Tenforde MW, DeCuir J, Payne AB.JAMA Netw Open. 2025 Jun 2;8(6):e2517402. doi: 10.1001/jamanetworkopen.2025.17402.
- Interim Estimates of 2024-2025 COVID-19 Vaccine Effectiveness Among Adults Aged ≥18 Years - VISION and IVY Networks, September 2024-January 2025.Link-Gelles R, Chickery S, Webber A, Ong TC, Rowley EAK, DeSilva MB, Dascomb K, Irving SA, Klein NP, Grannis SJ, Barron MA, Reese SE, McEvoy C, Sheffield T, Naleway AL, Zerbo O, Rogerson C, Self WH, Zhu Y, Lauring AS, Martin ET, Peltan ID, Ginde AA, Mohr NM, Gibbs KW, Hager DN, Prekker ME, Mohamed A, Johnson N, Steingrub JS, Khan A, Felzer JR, Duggal A, Wilson JG, Qadir N, Mallow C, Kwon JH, Columbus C, Vaughn IA, Safdar B, Mosier JM, Harris ES, Chappell JD, Halasa N, Johnson C, Natarajan K, Lewis NM, Ellington S, Reeves EL, DeCuir J, McMorrow M, Paden CR, Payne AB, Dawood FS, Surie D; CDC COVID-19 Vaccine Effectiveness Collaborators.MMWR Morb Mortal Wkly Rep. 2025 Feb 27;74(6):73-82. doi: 10.15585/mmwr.mm7406a1.PMID: 40014628
- Patterns in Prescribing and Dispensing Influenza Antivirals Among Adults With Influenza Presenting to Urgent Care and Emergency Department Settings: VISION Network, 2023-2024.Adams K, Garg S, Tartof SY, Irving SA, DeSilva MB, Klein NP, Natarajan K, Dascomb K, Grannis SJ, Ong TC, Salas SB, Sy LS, Lewin B, Qian L, Naleway AL, Koppolu PD, McEvoy CE, Akinsete O, Essien I, Fireman B, Zerbo O, Jacobson KB, Timbol J, Neelam V, Reeves EL, Dickerson M, Ray C, Link-Gelles R, Mak J, Ball SW, O'Reilly M, Olsen SJ, Tenforde MW.Clin Infect Dis. 2025 Nov 6;81(4):e172-e183. doi: 10.1093/cid/ciaf178.PMID: 40184184
2024
- Link-Gelles R, Rowley EAK, DeSilva M.B. et al Interim Effectiveness of Updated 2023-2024 (Monovalent XBB.1.5) COVID-19 Vaccines Against COVID-19-Associated Hospitalization Among Adults Aged ≥18 Years with Immunocompromising Conditions - VISION Network, September 2023-February 2024. MMWR. Morbidity and mortality weekly report 2024; 73(12), 271–276. https://doi.org/10.15585/mmwr.mm7312a5
- Levy ME, Yang D, Dunne MM, et al. Risk of COVID-19 Hospitalization and Protection Associated With mRNA Vaccination Among US Adults With Psychiatric Disorders. Influenza and Other Respiratory Viruses. 2024;18(3). doi:https://doi.org/10.1111/irv.13269
- DeCuir J, Payne AB, Self WH, et al. Interim effectiveness of updated 2023-2024 (Monovalent XBB.1.5) COVID-19 vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalization among immunocompetent adults aged ≥18 years - VISION and IVY Networks, September 2023-January 2024. MMWR. Morbidity and Mortality Weekly Report. 2024;73(8):256-262. https://www.cdc.gov/mmwr/volumes/73/wr/mm7308a5.htm
- Frutos AM, Price AM, Harker E, et al. Interim Estimates of 2023–24 Seasonal Influenza Vaccine Effectiveness — United States. MMWR Morbidity and Mortality Weekly Report. 2024;73. doi: https://doi.org/10.15585/mmwr.mm7308a3
- Respiratory syncytial virus (RSV) vaccine effectiveness against RSV-associated hospitalisations and emergency department encounters among adults aged 60 years and older in the USA, October, 2023, to March, 2024: a test-negative design analysis.Payne AB, Watts JA, Mitchell PK, Dascomb K, Irving SA, Klein NP, Grannis SJ, Ong TC, Ball SW, DeSilva MB, Natarajan K, Sheffield T, Bride D, Arndorfer J, Naleway AL, Koppolu P, Fireman B, Zerbo O, Timbol J, Goddard K, Dixon BE, Fadel WF, Rogerson C, Allen KS, Rao S, Mayer D, Barron M, Reese SE, Rowley EAK, Najdowski M, Ciesla AA, Mak J, Reeves EL, Akinsete OO, McEvoy CE, Essien IJ, Tenforde MW, Fleming-Dutra KE, Link-Gelles R.Lancet. 2024 Oct 19;404(10462):1547-1559. doi: 10.1016/S0140-6736(24)01738-0.PMID: 39426837
- Effectiveness of the Original Monovalent and Bivalent COVID-19 Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters in Pregnant Persons Who Were Not Immunocompromised: VISION Network, June 2022-August 2023.Avrich Ciesla A, Lazariu V, Dascomb K, Irving SA, Dixon BE, Gaglani M, Naleway AL, Grannis SJ, Ball S, Kharbanda AB, Vazquez-Benitez G, Klein NP, Natarajan K, Ong TC, Embi PJ, Fleming-Dutra KE, Link-Gelles R, Zerbo O.Open Forum Infect Dis. 2024 Aug 28;11(9):ofae481. doi: 10.1093/ofid/ofae481. eCollection 2024 Sep.PMID: 39286032
- Clinical Epidemiology and Risk Factors for Critical Outcomes Among Vaccinated and Unvaccinated Adults Hospitalized With COVID-19-VISION Network, 10 States, June 2021-March 2023.Griggs EP, Mitchell PK, Lazariu V, Gaglani M, McEvoy C, Klein NP, Valvi NR, Irving SA, Kojima N, Stenehjem E, Crane B, Rao S, Grannis SJ, Embi PJ, Kharbanda AB, Ong TC, Natarajan K, Dascomb K, Naleway AL, Bassett E, DeSilva MB, Dickerson M, Konatham D, Fireman B, Allen KS, Barron MA, Beaton M, Arndorfer J, Vazquez-Benitez G, Garg S, Murthy K, Goddard K, Dixon BE, Han J, Grisel N, Raiyani C, Lewis N, Fadel WF, Stockwell MS, Mamawala M, Hansen J, Zerbo O, Patel P, Link-Gelles R, Adams K, Tenforde MW.Clin Infect Dis. 2024 Feb 17;78(2):338-348. doi: 10.1093/cid/ciad505.PMID: 37633258
2023
- Adams K, Weber ZA, Yang DH, et al. Vaccine Effectiveness Against Pediatric Influenza-A–Associated Urgent Care, Emergency Department, and Hospital Encounters During the 2022–2023 Season: VISION Network. Clinical Infectious Diseases. 2023;78(3):746-755. doi:https://doi.org/10.1093/cid/ciad704
- DeSilva MB, Knowlton G, Rai, NK. et al. Vaccine effectiveness against SARS-CoV-2 related hospitalizations in people who had experienced homelessness or incarceration - Findings from the Minnesota EHR Consortium. Journal of Community Health. 2023;48(4):610-617. https://link.springer.com/article/10.1007/s10900-023-01308-3
- Payne AB, Ciesla AA, Rowley EAK, et al. Impact of accounting for correlation between COVID-19 and influenza vaccination in a COVID-19 vaccine effectiveness evaluation using a test-negative design. Vaccine. 2023;41(48):7181-7188. https://www.sciencedirect.com/science/article/pii/S0264410X23013373?via%3Dihub
- Patel P, Schrader KE, Rice CE, et al. Effectiveness of the original monovalent coronavirus disease 2019 vaccines in preventing emergency department or urgent care encounters and hospitalizations among adults with disabilities: VISION Network, June 2021-September 2022. Open Forum Infectious Diseases. 2023;10(11) https://academic.oup.com/ofid/article/10/11/ofad474/7286130
- Griggs EP, Mitchell PK, Lazariu V, et al. Clinical epidemiology and risk factors for critical outcomes among vaccinated and unvaccinated adults hospitalized with COVID-19-VISION Network, 10 States, June 2021-March 2023. Clinical Infectious Diseases. 2023;78(2):338-347. https://academic.oup.com/cid/article/78/2/338/7252326
- Link-Gelles R, Ciesla AA, Rowley EA, et al. Effectiveness of monovalent and bivalent mRNA vaccines in preventing COVID-19-associated emergency department and urgent care encounters among children aged 6 months-5 years - VISION Network, United States, July 2022-June 2023. MMWR. Morbidity and Mortality Weekly Report. 2023;72(33):998-1003. https://www.cdc.gov/mmwr/volumes/72/wr/mm7233a2.htm
- Link-Gelles R, Levy ME, Natarajan K, et al. Estimation of COVID-19 mRNA vaccine effectiveness and COVID-19 illness and severity by vaccination status during Omicron BA.4 and BA.5 sublineage periods. JAMA Network Open. 2023;6(6) https://doi.org/10.1001/jamanetworkopen.2023.2598
- Bozio CH, Butterfield KA, Briggs Hagen M et al. Protection from COVID-19 mRNA vaccination and prior SARS-CoV-2 infection against COVID-19-associated encounters in adults during Delta and Omicron predominance. Journal of Infectious Diseases. 2023;227(12):1348-1357. https://academic.oup.com/jid/article/227/12/1348/7045997
- Embi PJ, Levy ME, Patel P, et al. Effectiveness of COVID-19 vaccines at preventing emergency department or urgent care encounters and hospitalizations among immunocompromised adults: An observational study of real-world data across 10 US states from August-December 2021. Vaccine. 2023;41(22):3411-3418. https://doi.org/10.1016/j.vaccine.2023.05.038
- Klein NP, Demarco M, Fleming-Dutra KE, et al. Effectiveness of BNT162b2 COVID-19 vaccination in children and adolescents. Pediatrics. 2023;150(2)https://doi.org/10.1542/peds.2022-060894
- Link-Gelles R, Weber ZA, Reese SE, et al. Estimates of bivalent mRNA vaccine durability in preventing COVID-19-associated hospitalization and critical illness among adults with and without immunocompromising conditions - VISION Network, September 2022-April 2023. MMWR. Morbidity and Mortality Weekly Report. 2023;72(21):545-550. https://www.cdc.gov/mmwr/volumes/72/wr/mm7221a3.htm?s_cid=mm7221a3_w
- Adams K, Riddles JJ, Rowley EAK, et al. Number needed to vaccinate with a COVID-19 booster to prevent a COVID-19-associated hospitalization during SARS-CoV-2 Omicron BA.1 variant predominance, December 2021–February 2022, VISION Network: a retrospective cohort study. The Lancet. 2023;5(3) https://doi.org/10.1016/j.lana.2023.100530
- Dalton AF, Weber ZA, Allen KS, et al. Relationships between social vulnerability and COVID-19 vaccination coverage and vaccine effectiveness. Clinical Infectious Diseases. 2023;76(9): 1615-1625 https://doi.org/10.1093/cid/ciad003
- Tenforde MW, Weber ZA, DeSilva MB, et al. Vaccine Effectiveness Against Influenza-Associated Urgent Care, Emergency Department, and Hospital Encounters During the 2021–2022 Season, VISION Network. The journal of infectious diseases (Online University of Chicago Press)/The Journal of infectious diseases. 2023;228(2):185-195. doi:https://doi.org/10.1093/infdis/jiad015
- Tenforde MW, Weber ZA, Yang DH, et al. Influenza Vaccine Effectiveness Against Influenza A–Associated Emergency Department, Urgent Care, and Hospitalization Encounters Among US Adults, 2022–2023, The Journal of Infectious Diseases, Volume 230, Issue 1, 15 July 2024, Pages 141–151, https://doi.org/10.1093/infdis/jiad542
- Rao S, Bozio C, Butterfield K, et al. Accuracy of COVID-19-Like-Illness Diagnoses in Electronic Health Record Data: Retrospective Cohort Study (Preprint). JMIR Formative Research. Published online May 3, 2022;7:e39231 doi:https://doi.org/10.2196/39231
2022
- Tenforde MW, Weber ZA, Natarajan K, et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19–associated emergency department or urgent care encounters and hospitalizations among immunocompetent adults — VISION Network, nine states, September–November 2022. MMWR. Morbidity and Mortality Weekly Report. 2022;71(51-52):1653-1658. https://www.cdc.gov/mmwr/volumes/71/wr/mm715152e1.htm
- Ferdinands J M, Rao S, Dixon B E, et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022;379 https://www.bmj.com/content/379/bmj-2022-072141
- Britton A, Embi PJ, Levy ME, et al. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalizations among immunocompromised adults during SARS-CoV-2 Omicron predominance — VISION Network, 10 states, December 2021— August 2022. MMWR. Morbidity and Mortality Weekly Report. 2022;71(42):1328-1333. https://www.cdc.gov/mmwr/volumes/71/wr/mm7142a4.htm?s_cid=mm7142a4_w
- DeSilva MB, Mitchell PK, Klein NP, et al. Protection of 2 and 3 mRNA vaccine doses against severe outcomes among adults hospitalized with COVID-19 – VISION Network, August 2021 – March 2022. Journal of Infectious Diseases. Advance online publication. https://doi.org/10.1093/infdis/jiac458
- Schrag SJ, Verani JR, Dixon BE, et al. Estimation of COVID-19 mRNA vaccine effectiveness against medically attended COVID-19 in pregnancy during periods of Delta and Omicron variant predominance in the United States. JAMA Network Open. 2022;5(10) https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2796667?resultClick=1
- Link-Gelles R, Levy ME, Gaglani M, et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 sublineages predominated — VISION Network, 10 states, December 2021–June 2022. MMWR. Morbidity and Mortality Weekly Report. 2022;71(29):925-933. https://www.cdc.gov/mmwr/volumes/71/wr/mm7129e1.htm?s_cid=mm7129e1_w
- Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19–associated emergency department and urgent care encounters and hospitalizations among non-immunocompromised children and adolescents aged 5–17 years — VISION Network, 10 states, April 2021–January 2022. MMWR. Morbidity and Mortality Weekly Report. 2022;71(9):352-358. https://www.cdc.gov/mmwr/volumes/71/wr/mm7109e3.htm?s_cid=mm7109e3_w
- Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR. Morbidity and Mortality Weekly Report. 2022;71(7):255-263. https://www.cdc.gov/mmwr/volumes/71/wr/mm7107e2.htm?s_cid=mm7107e2_w
- Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance — VISION network, 10 states, August 2021–January 2022. MMWR. Morbidity and Mortality Weekly Report. 2022;71(4):139-145. https://www.cdc.gov/mmwr/volumes/71/wr/mm7104e3.htm?s_cid=mm7104e3_w
- Natarajan K, Prasad N, Dascomb K, et al. Effectiveness of homologous and heterologous COVID-19 booster doses following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) vaccine dose against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults — VISION Network, 10 states, December 2021–March 2022. MMWR. Morbidity and Mortality Weekly Report. 2022;71(13):495-502. https://www.cdc.gov/mmwr/volumes/71/wr/mm7113e2.htm?s_cid=mm7113e2_w
- Bozio CH, Butterfield K, Irving SA, et al. Relative Risks of COVID-19-Associated Hospitalizations and Clinical Outcomes by Age and Race/Ethnicity-March 2020-March 2021. Open Forum Infectious Diseases. 2022;9(10):ofac376. doi:https://doi.org/10.1093/ofid/ofac376
2021
- Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19–associated hospitalizations among immunocompromised adults — Nine states, January–September 2021. MMWR. Morbidity and Mortality Weekly Report. 2021;70(44):1553-1559. https://www.cdc.gov/mmwr/volumes/70/wr/mm7044e3.htm?s_cid=mm7044e3_w
- Grannis SJ, Rowley EA, Ong TC, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19–associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance — Nine states, June–August 2021. MMWR. Morbidity and Mortality Weekly Report. 2021;70(37):1270-1273. https://www.cdc.gov/mmwr/volumes/70/wr/mm7037e2.htm
- Thompson M, Stenehjem E, Grannis SJ, et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. New England Journal of Medicine. 2021; 385(15): 1355-1371. https://doi.org/10.1056/NEJMoa2110362
- Bozio CH, Grannis SJ, Naleway AL, et al. Laboratory-Confirmed COVID-19 Among Adults Hospitalized with COVID-19–Like Illness with Infection-Induced or mRNA Vaccine-Induced SARS-CoV-2 Immunity — Nine States, January–September 2021. MMWR Morbidity and Mortality Weekly Report. 2021;70(44). doi:https://doi.org/10.15585/mmwr.mm7044e1
Resources
- More information about how CDC's VE studies are conducted and how to interpret results is available: How Flu Vaccine Effectiveness and Efficacy are Measured.
- Seasonal Influenza Vaccine Effectiveness provides tables that show the overall adjusted VE and related references for each season starting in 2004-2005.
