At a glance
Chapter 18 of The CDC Field Epidemiology Manual
Introduction
The term healthcare setting represents a broad array of services and places where healthcare occurs, including acute care hospitals, urgent care centers, rehabilitation centers, nursing homes and other long-term care facilities, specialized outpatient services (e.g., hemodialysis, dentistry, podiatry, chemotherapy, endoscopy, and pain management clinics), and outpatient surgery centers. In addition, some healthcare services are provided in private offices or homes.
Within each type of setting, specific locations or services might be the focal point of an epidemiologic investigation. Acute care hospitals are complex organizations that can have multiple specialized areas for triage and emergency care, inpatient and outpatient surgical procedures, management of immunosuppressed populations (e.g., oncology or transplant recipients), rehabilitation services, and intensive care units. An understanding of the types of patients and clinical services provided in a given setting is crucial for recognizing infectious disease transmission risks. Problems identified within a healthcare setting also can be related to use of medications or devices that became contaminated at the point of manufacture or other locations outside the setting of interest.
This chapter is an overview of outbreak investigations in healthcare settings. Although most reported outbreaks in healthcare settings are caused by infections, outbreaks also can be associated with exposures to noninfectious chemical and other toxic agents. This chapter mainly addresses epidemiologic investigations of infections but includes some noninfectious disease examples as well.
Context for Infections Associated with Healthcare Settings
Defining Healthcare-Associated Infections (HAIs)
Healthcare-associated infections (HAIs) are one of the leading causes of unnecessary death and avoidable harm for patients receiving medical care. They are a serious threat to public health, and each year millions of patients are affected by HAIs worldwide.
An HAI is an infection associated with healthcare delivery in any setting. This term reflects the inability to always determine with certainty where the pathogen is acquired because patients might be colonized (i.e., microorganisms on or in a person without causing a disease) or exposed outside the healthcare setting, and patients frequently move among different settings within a healthcare system1. HAIs might appear after discharge, and HAIs transmission can involve visitors and healthcare personnel (HCP) in addition to patients.
HAIs Causes
During the course of receiving medical care, patients are exposed to different microorganisms, and infectious agents can be acquired from
- Infected or colonized HCP or another patient (cross-infection);
- The patient's own microbiome (endogenous infection);
- Environmental surfaces or objects contaminated from another human (e.g., bed rails, intravenous poles, countertops, or bathroom surfaces);
- Contaminated medical devices (e.g., central venous catheters, urinary catheters, endoscopes, surgical instruments, or ventilators);
- Contaminated medications;
- Contaminated water sources; or
- Air from heating, ventilation, or air-conditioning systems.
A vast number of agents have been implicated in HAIs transmission scenarios; these include a constantly evolving list of bacteria, fungi, viruses, parasites, and prions. HAIs outbreaks can be caused by pathogens that are common in the community or by pathogens that are rarely observed outside of healthcare environments and specific patient populations. The likelihood of infection after exposure is related to1 the characteristics of the microorganisms, including resistance to antimicrobial agents, intrinsic virulence, and amount of infective material;2 patient factors, including immune status, wounds, other underlying comorbidities, duration of care, prior antimicrobial exposures, and whether their care involves surgical or other invasive procedures or devices; and 3facility-level factors, including inattention to individual or environmental hygiene, crowding, lack of an effective infection control program, and shortage of trained infection control practitioners.
HAIs Prevalence
Despite progress during the past decade in preventing certain types of HAIs through improved surveillance and infection prevention and control practices, HAIs remain common. The Centers for Disease Control and Prevention's (CDC) HAI Prevalence Survey of 2014 regarding the burden of HAIs in US hospitals reported that, during 2011, an estimated 722,000 HAIs occurred in US acute care hospitals (Table 18.1)2. Additionally, approximately 75,000 patients with HAIs died during their hospitalizations. More than half of all HAIs occurred outside of intensive care units2.
Table 18.1
Overview of Sequence of Steps Involved in an HAI Investigation
Typically, the state, territorial, local or tribal public health authority is notified of a potential HAI outbreak by a healthcare provider or facility on the basis of laboratory or other HAIs surveillance data or as a result of recognizing an unusual infection cluster. Health departments might also detect potential outbreaks through surveillance or after being directly contacted by affected patients. The public health department can contact CDC for additional technical assistance. In certain instances, however, the healthcare facility's administrator contacts CDC directly; in that instance, CDC subject matter experts can provide technical advice, but they must coordinate with the state or local public health department before becoming involved in a field investigation. Depending on the scenario, initial steps taken by a public health authority might include the following:
- Public health department epidemiologists gather information and provide consultation to the healthcare facility reporting the potential outbreak.
- The public health department official begins an on-site investigation and considers inviting CDC to assist.
- The public health personnel and healthcare facility staff gather and analyze information through interviews, chart reviews, observations, and environmental sampling to identify a point source or practice that might have caused the outbreak.
- The public health personnel recommend new or revised measures to stop the outbreak and prevent additional HAIs.
Field Investigation
The basic steps of epidemiologic field investigations that are described in Chapter 3 are adapted here for investigations in healthcare settings.
- Step 1. Verify the diagnosis.
- Step 2. Confirm presence of an HAI outbreak.
- Step 3. Alert key partners about the investigation.
- Step 4. Establish case definition(s).
- Step 5. Identify and count cases.
- Step 6. Organize data according to person, place, time, and size.
- Step 7. Conduct targeted observations, review key concerns with setting healthcare providers, and develop abstraction forms.
- Step 8. Formulate and test hypotheses.
- Step 9. Infection control assessment and implementation of control measures.
- Step 10. Follow-up, communicate findings, and notify patients.
Step 1. Verify the Diagnosis
Early in the investigation, identify as accurately as possible the specific nature of the disease by
- Ensuring that the diagnosis is correct;
- Evaluating for possible laboratory error as the basis for increased diagnoses;
- Evaluating possible changes in surveillance and case definitions; and
- Reviewing clinical findings and microbiological testing results.
Step 2. Confirm Presence of an HAI Outbreak
- An early major step in the investigation is verifying that a suspected outbreak is real. Cases in excess of historical or predicted levels might not necessarily indicate an outbreak.
- Some cases might be part of an actual outbreak with a common cause, whereas others might be unrelated.
- Reporting might be increased because of changes in local reporting procedures, changes in the case definition, increased interest reflecting local or national awareness, or improvements or other changes in diagnostic procedures.
- Some cases might be part of an actual outbreak with a common cause, whereas others might be unrelated.
- Possible community-associated or other explanations for illness not associated with healthcare should be investigated. Public health surveillance data sometimes can inform investigators about an increase in infections that is initially recognized in healthcare settings but actually is part of a broader community outbreak.
- Pseudo-outbreaks (e.g., those caused by laboratory processing errors or contamination of clinical diagnostic equipment, such as bronchoscopes, without clinical illness) are important to investigate and control because they can lead to unnecessary antibiotic prescriptions, diagnostic procedures, and other potentially harmful interventions to patients. Pseudo-outbreaks also represent opportunities to recognize and correct inadequate infection control processes (e.g., device reprocessing).
Step 3. Alert Key Partners About the Investigation
After confirming an HAI outbreak, investigators should inform key partners.
- Include relevant facility staff (e.g., hospital epidemiologist, infection control practitioner, environmental services department staff, medical staff, administrative leaders, media relations director, and department leads for the affected facility area).
- Ask the clinical laboratory director to save all isolates that might be related to the outbreak.
- Notify local, state, national, and international public health officials, as required.
- Notify regulatory partners (e.g., Food and Drug Administration, Environmental Protection Agency) if the investigation involves regulated medical devices or products.
- Notify professional oversight organizations, as required (e.g., pharmacy boards, clinician licensing boards).
Step 4. Establish Case Definitions
A case definition is used to identify persons who are, or might be, infected and to characterize them in relation to the disease, time and location of exposure or illness onset, and other persons affected. A case definition usually includes
- Clinical information about the disease (e.g., laboratory test results, symptoms, and signs);
- Demographic characteristics of affected patients (e.g., age, race/ethnicity, sex, and occupation);
- Information about the location of possible exposure or time of onset (e.g., what part of an intensive care unit, radiology suite, operating room, ward, or other unit); and
- A defined time during which exposure or onset occurred.
Ideally, the case definition initially should be broad enough to include most if not all cases; it can then be refined as the investigation progresses and more relevant information is accumulated.
The case definition also should be based on the etiologic agent, if known, and can include clinically infected and colonized patients. The specificity of the definition can vary.
- A stratified case definition (e.g., confirmed vs. probable vs. possible [i.e., suspected], or confirmed vs. probable) can be applied to account for the uncertainty of certain diagnoses.
- Confirmed: Usually must have laboratory verification.
- Probable: Usually has typical clinical features and an epidemiologic link to confirmed cases but lacks laboratory confirmation.
- Possible: Usually has fewer of the typical clinical features or weaker epidemiologic links to confirmed cases.
The following are example case definitions:
- A methicillin-resistant Staphylococcus aureus bloodstream infection in a patient in Hospital A's neonatal intensive care unit during January 1–December 31.
- Isolation of Burkholderia cepacia complex matching the outbreak strain in a hospitalized patient who received Medication A any time during January 1–June 30.
- Fever (temperature >38.5ºC) and compatible symptoms in a patient who had been in an Ebola virus infection–affected country 21 days or less before symptom onset.
Step 5. Identify and Count Cases
Outbreaks often are first recognized and reported by perceptive HCP or identified during surveillance activities. Additional cases related to the outbreak can be identified through multiple types of data and records, for example,
- Central service or supply records,
- Occupational health records,
- Hospital billing records,
- Operative notes,
- Infection control assessment,
- Pathology reports,
- Interviews with physicians,
- Pharmacy reports,
- Log books,
- Purchasing records,
- Medical records,
- Radiology reports,
- Microbiology data, and
- Surveillance records.
Step 6. Organize Data According to Person, Place, Time, and Size
Step 6.1. Create a Line Listing
The line listing, which typically involves using a spreadsheet program so that data can be sorted easily during data analysis, helps guide the outbreak investigation and permits rapid examination of exposures. For each case, collect and array the following types of information encompassed by the case definition:
- Location information. Location within the facility (e.g., room number, bed number, and adjacent rooms).
- Demographic information. Typically, age, sex, race/ethnicity, and occupation, plus other relevant characteristics of the affected population or others at risk.
- Clinical information. Symptoms, signs, and laboratory tests (e.g., culture, serology, or polymerase chain reaction results).
- Risk factor information. Adjust the investigation to the specific disease in question.
Develop a standard questionnaire if patients are to be contacted and interviewed. Example Data to Obtain for a Line Listing summarizes data that should be obtained for a line listing in an HAI investigation (see below).
Step 6.2. Construct an Epidemic Curve
Create an epidemic curve (epi curve) to visually demonstrate the outbreak's magnitude and time course. The epidemic curve
- Illustrates the course of the epidemic by day, week, or month and can help project its forward trajectory;
- Might help estimate a probable exposure period and, therefore, focus a questionnaire on that period, especially when an approximate incubation period is known or suspected.
- Might enable inferences about the epidemic pattern (e.g., whether common source or person-to-person).
In the example (Figure 18.1), confirmed and probable cases are plotted over time to show the onset of adverse events associated with a contaminated medical product, including markers for key events during the investigation. This example was adapted after a published field investigation4.
Example Data to Obtain for a Line Listing
- Patient characteristics (e.g., age, sex, race/ethnicity, comorbidities, birthweight)
- Date of admission
- Date of illness onset
- Date of discharge
- Facility locations/units (i.e., room number, bed, and adjoining room numbers)
- Medications
- Procedures
- Consults (e.g., laboratory or nursing)
- Attending healthcare personnel (e.g., specific nursing staff, respiratory therapists, and physicians)
Collect the information described previously on a standard case-report form, questionnaire, or data abstraction form (Table 18.2).
- Abstract selected key items to build a table. Each column represents a variable, and each row represents a case.
- Add new cases as they are identified. This simple format allows the investigator to scan key information on every case and to update it easily.
An example HAI outbreak abstraction form and user guide are available with the Healthcare-Associated Infection Outbreak Investigation Toolkit3.
Figure 18.1
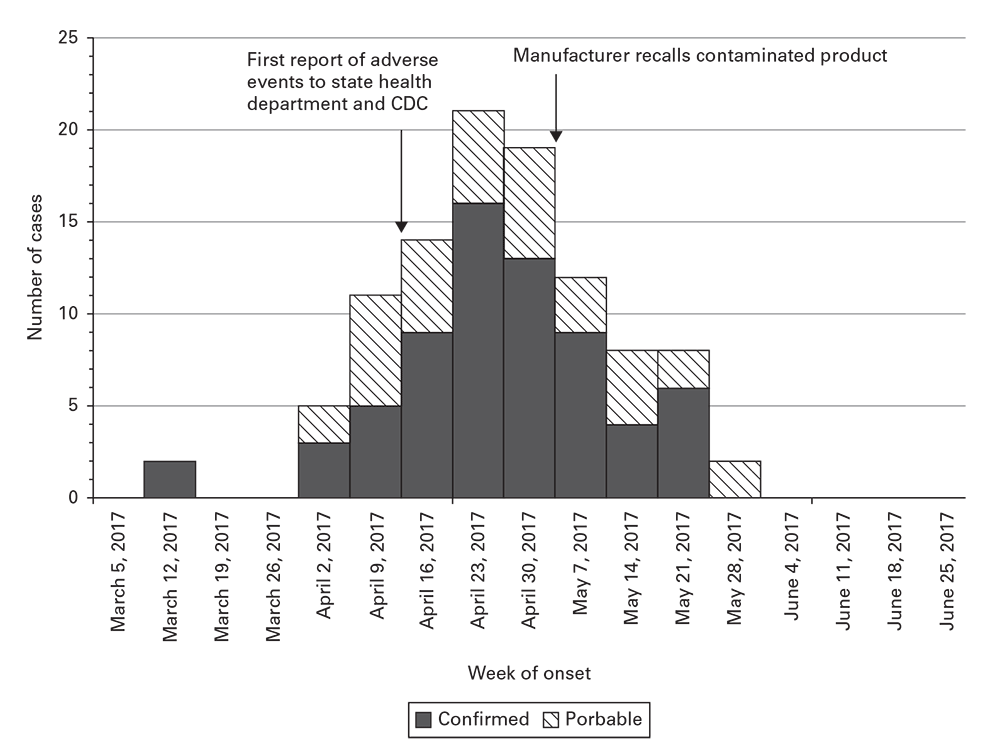
Source: Adapted from Reference4.
Table 18.2
Example line listing for healthcare-associated infection investigations
ICU, intensive care unit; HIV, human immunodeficiency virus.
Step 7. Conduct Targeted Observations, Review Key Concerns with Setting Healthcare Providers, and Develop Abstraction Forms
- Healthcare setting investigations often are complex and hypotheses elusive. Most outbreaks are solved through rigorous observation and discussion of procedural concerns with facilities' HCP.
- Focus on whether actual practices deviate from recommended infection control practices and the facility's policies. Such discrepancies are best identified through a combination of direct observations and review of HCP self-reported practices.
- Examine whether practices differ among HCP.
- Review recent scientific literature related to the key concerns involved with the outbreak.
- Observe key activities (e.g., medication preparation, care of vascular access, hand hygiene, adherence to isolation precautions, device and equipment reprocessing, environmental services, and respiratory therapy) related to suspicions about likely transmission pathways that might be involved in the outbreak.
- In addition to available local and setting-specific tools, use general infection control assessment tools available through CDC to assist with both prevention programs and response scenarios5.
- Focus on whether actual practices deviate from recommended infection control practices and the facility's policies. Such discrepancies are best identified through a combination of direct observations and review of HCP self-reported practices.
- Review key concerns with facility HCP to help generate hypotheses about the source and mode(s) of transmission.
- Are protocols accurate and up-to-date?
- How does actual practice compare with written or verbal protocols?
- Are procedures consistently adherent to protocols?
- Do instances exist where procedures must be performed differently?
- Have other HCP been observed to perform procedures differently from protocol?
- What are the challenges with maintaining accurate and consistent techniques?
- What do you think is the root cause of the outbreak?
- What procedures or medications might not be documented in the medical record?
- Is all information in patients' medical records accurate and current?
- Are protocols accurate and up-to-date?
- Develop, modify if necessary, and complete abstraction forms. Abstraction forms can include additional details about patients' illnesses that provide information for analytic studies. Abstraction forms can be adapted from information collected on the line listing to help determine which fields or sections of the abstraction form to include.
Step 8. Formulate and Test Hypotheses
Step 8.1. Conduct Analytic Studies
A case–control study is the approach most commonly used for hypothesis testing for field investigations in healthcare settings. The frequency of exposure to a risk factor among a group of case-patients (i.e., persons with the HAIs) is compared with the frequency of exposure to that risk factor among a group of controls (i.e., persons without the HAIs). Controls must be selected carefully to limit bias; for example, two or more controls for each case-patient might be needed to provide sufficient statistical power. Cohort studies might also be useful in HAIs investigations.
However, analytic studies are labor-intensive and in healthcare settings not always necessary to identify the likely source of an outbreak and to institute control measures. For example, a combination of laboratory evidence and observations of serious lapses in infection control practices that are known to be associated with transmission are frequently sufficient to recommend and implement control measures. The following considerations can influence the decision to conduct an analytic study:
- Will an analytic study add to what is already known about the cause of the outbreak or contribute to the control recommendations?
- Is the necessary technical and statistical support available?
- Is the number of cases large enough to support statistical inferences?
- Can enough controls be selected to minimize bias?
- Is information available for testing possible risk factors?
Step 8.2. Conduct Environmental Sampling and Testing
A major tool available for HAIs investigations is environmental sampling and laboratory analysis. An environmental sampling strategy (i.e., where and what should be cultured) should always be influenced by epidemiologic findings. Molecular methods (e.g., polymerase chain reaction or pulsed-field gel electrophoresis) can be deployed in certain investigations to link environmental samples to clinical specimens. Optimal methods should be discussed with laboratory personnel experienced in environmental sampling to determine how specimens should be obtained and where the cultures can be processed. Often, public health laboratories are needed to support specialized sampling of surfaces, devices, water, or air or when substantial numbers of samples should be obtained. A plan for correctly processing and interpreting results should be established before sample collection. When developing a sampling plan, specify the following:
- Ensure protocols are in place for safe and correct collection and processing of environmental samples. Many environmental pathogens need special procedures for collection, handling, storage, and media for culture growth. Contamination of samples is possible if procedures are inadequate, and overgrowth of organisms in the samples can obscure results for the pathogen of interest. Safety precautions for personnel collecting the samples also need to be followed.
- Use epidemiologic findings to guide testing so that laboratory resources are used appropriately and results can be meaningfully interpreted. Interpretation of results should consider the following:
- Positive environmental samples are not necessarily evidence of transmission from a particular source; thus, understanding how patients are most likely being exposed to the organism from the environment is crucial. Organisms can be polyclonal even from the same source; for example, biofilms in plumbing systems frequently have many different species and strains of coexisting microorganisms.
- Conversely, negative environmental samples do not rule out that the pathogen of interest is present or was present at the time of transmission.
- Samples that test negative must be confirmed to be true-negatives and not inactivated by environmental disinfectants or media preservatives.
- Laboratory analyses (e.g., pulsed-field gel electrophoresis for DNA fingerprinting) can be used to determine whether environmental isolates match those from patients; however, organisms can be polyclonal.
- Positive environmental samples are not necessarily evidence of transmission from a particular source; thus, understanding how patients are most likely being exposed to the organism from the environment is crucial. Organisms can be polyclonal even from the same source; for example, biofilms in plumbing systems frequently have many different species and strains of coexisting microorganisms.
- Even with correct methods and materials, sensitivity can be low, and negative results do not necessarily rule out environmental reservoirs.
Analytic studies can support a hypothesis even if a source cannot be confirmed by environmental testing.
Step 8.3. Considerations for Testing of HCP
Testing HCP can further support or confirm possible associations between HCP colonization and infection transmission to patients. These scenarios are most readily recognized in point-source outbreaks involving colonized HCP and absence of other clear links among infected patients. Possible transmission from HCP to patients should be considered in the context of the type of organism and investigation into other possible transmission routes.
- HCP testing should only be undertaken after careful consideration of (1) how the results will help control the outbreak, (2) what duty or work restrictions might need to be applied, and (3) a known decolonization or other specific control strategy to undertake for personnel who test positive.
- Testing of HCP provokes anxiety, and positive results can be highly stigmatizing. The rationale for testing should be clearly explained to HCP, and strict discretion should be emphasized when obtaining samples and communicating results.
- Positive results should not necessarily be regarded as evidence of causality because HCP frequently acquire microorganisms from infected patients.
- Because of limitations in the sensitivity of cultures and the potential for transient contamination, negative results can be reassuring in certain scenarios but should not be regarded as excluding the possibility that HCP were involved in transmission.
Step 9. Infection Control Assessment and Implementation of Control Measures
Step 9.1. Infection Control Assessment
When investigating an HAI outbreak, an understanding of infection prevention and control is crucial to determine which control measures need to be implemented. A setting-specific infection control assessment tool can help accomplish this task56. Such tools provide a framework for assessing major areas of infection control and help guide a facility infection control assessment. Infection Control Domains for Assessment summarizes major infection control domains to consider when performing an assessment (see section below). A physical walkthrough of the specific healthcare setting should be targeted for specific domains, depending on the hypothesized source of transmission (i.e., care locations or others areas hypothesized to be involved in the outbreak), including
- Triage and emergency care departments,
- Inpatient areas,
- Device reprocessing and storage areas,
- On-site or off-site compounding pharmacies,
- Operating or other procedure rooms, or
- Management areas for specific equipment (e.g., hemodialysis machines or ventilators).
Infection Control Domains for Assessment
- Infection control program review
- Infection control training
- Hand hygiene
- Personal protective equipment use, availability, quality, and training
- Prevention of catheter-associated urinary tract infection
- Prevention of central line–associated bloodstream infection
- Prevention of ventilator-associated event
- Injection safety
- Prevention of surgical site infection
- Prevention of Clostridium difficile infection
- Environmental cleaning
- Waste management
- Device reprocessing
- Multidrug-resistant organism surveillance
Step 9.2. Defining Infection Control Measures
Control measures should be implemented as soon as deficiencies or gaps are identified; these should be aimed at specific links in the infection chain, the agent, the source, or the reservoir. Multiple control measures might be required.
Ultimately, the primary goal is to stop transmission, even when the specific source remains unidentified. Therefore, implementing multiple control measures targeting different possibilities based on the initial observations might be necessary. Table 18.3 provides key examples of immediate control measures that can be used to manage an outbreak.
Certain new or targeted multidrug-resistant organisms warrant consultation with public health departments, and control measures can extend into the community or across healthcare systems. Control measures might include contact tracing, lower thresholds for screening patients and HCP, specialized environmental testing, and implementing systems to adhere to contact precautions or enhanced environmental cleaning and disinfection. A tiered approach for investigating and controlling transmission of such pathogens also might be needed7.
Step 10. Follow-Up, Communicate Findings, and Notify Patients
Step 10.1. Stages of the Follow-Up Investigation
- Refining the case definition. Refine the case definition on the basis of data gathered from initial case-patients, controls, and HCP. Capturing all cases and optimizing the power of analytic studies might require narrowing or expansion of the definition.
- Continuing case finding and surveillance. Continue case finding and surveillance efforts on the basis of the refined case definition. Surveillance should continue, for example, for 1 month, 3 months, or even 1 year (e.g., in a long-term care facility) after the outbreak to ensure it has ended.
- Reviewing control measures. Assess adherence and determine whether control measures need to be further enhanced or relaxed.
Table 18.3
Immediate control measures for outbreak management
Cross-transmission (transmission between persons)
Patient isolation and transmission-based precautions determined by infectious agent(s) Certain scenarios might require closure of locations to new admissions
Hand transmission
Improvements in handwashing and nonsterile glove use where needed
Airborne infections (e.g., tuberculosis or emerging viral pathogens)
Triage, detection, and patient isolation with recommended ventilation type (positive or negative air pressure)
Agent present in water, waterborne agent
Assessment of premise water system, liquid products, or medications Use of disposable devices where reusable equipment is suspected
Foodborne agent
Elimination of the suspected food
Environmental reservoirs
Review and enhancement, as needed, of cleaning and disinfection processes Interruption of suspected mode of delivery from environment to patient
Colonized or infected healthcare personnel
Review of facility policies and discussion of work restrictions, duty exclusions, treatment, personal hygiene, or other steps
High-risk infection control breaches for risk of bloodborne or other pathogen transmission
Immediate cessation of risky practice until corrective action can be instituted Patient notification Assurance that occupational health staff are aware
Step 10.2. Communication of Findings
Findings should be communicated to all partners involved in the investigation. This communication typically takes two forms: (1) an oral briefing for local health authorities and (2) a written report (e.g., for CDC or the state or local health department). The final report, which might await laboratory confirmation, should describe (1) the outbreak characteristics, (2) infection control problems that most likely contributed to the outbreak, and (3) any interventions that were instituted and their effects. Additionally, the report should make recommendations for preventing future occurrences8.
Step 10.3. Patient Notification
Notification of patients potentially exposed to infectious organisms and their healthcare providers should be considered during investigation of HAIs outbreaks, cases involving pathogens of public health concern, or unsafe infection prevention and control practices. Although the circumstances of each outbreak and infection control breach vary, communication needs during notifications are more predictable. CDC has published considerations for when to notify patients and a patient notification toolkit to support HCP and public health personnel throughout the notification process9.
Depending on the scenario, typical reasons for conducting notifications can include one or more of the following:
- Identify potentially exposed or infected patients who will derive a health benefit through follow-up testing or other clinical evaluation.
- Establish transparency between healthcare providers and patients and other stakeholders.
- Limit the spread of multidrug-resistant organisms or other pathogens of public health concern by identifying exposed patients and their contacts who should be managed under recommended precautions.
- Improve case finding by informing patients and providers about the outbreak and associated exposures and clinical signs and symptoms that might signify the infection of interest.
- Use the notification scenario as an educational and prevention opportunity by reminding healthcare providers about the importance of infection prevention and control.
Examples of recently conducted patient notification scenarios include lapses in injection safety, drug diversion, contact with other patients with drug-resistant organisms, and exposure to contaminated or incorrectly processed devices (e.g., cardiopulmonary bypass heater-cooler units, endoscopes or surgical instruments, and exposure to contaminated medications).
The objectives of patient notifications are to deliver a consistent message quickly to all affected patients and to inform patients about testing or other follow-up actions that should be taken. Major steps include
- Verifying that exposures have occurred and confirming the type of procedure or substances involved;
- Determining the timeframe of the breach and the number of patients potentially exposed;
- Determining the severity of potential risks to patients;
- Determining whether testing or other further evaluation is available or warranted;
- Identifying any options for prophylactic treatment of exposed persons; and
- Determining how and what entity will provide the initial and follow-up care if testing, evaluation, or postexposure prophylaxis are to be offered.
Guidance for assessing unsafe injection practices and other serious infection control breaches includes how to assess whether a breach warrants patient notification and a sample notification letters and other materials1011.
Step 10.4. Legal Concerns
HAIs outbreaks can result in litigation and have broad financial and public relations implications for affected facilities. This concern often increases the scrutiny and number of interested stakeholders in the investigation. Pressure might be applied not only to investigate rapidly, but also to implement necessary control strategies quickly. Additionally, public health records of HAIs outbreak responses frequently are the subject of Freedom of Information Act requests. Investigators should keep records of all steps taken, exercise care and discretion in how emails and other communications are used, and assume that any investigation records might become publicly available or used as part of litigation proceedings.
Common Outbreak Settings and Response
Wide variability exists across types of healthcare settings with regard to patient susceptibility to infection, infectious exposures, types of healthcare services provided, and pathogenic organisms likely to be present. Additionally, care locations can differ within the same facility. For example, acute care hospitals often have operating rooms, neonatal intensive care units, oncology wards, and burn units. Understanding these different settings when investigating HAIs outbreaks is crucial. Tables 18.4 and 18.5 summarize some of the common outbreak and exposure scenarios that result during epidemiologic consultations with health facility managers.
Table 18.4
Examples of cross-cutting outbreaks across multiple types of healthcare settings
Any
Infected or colonized persons (healthcare personnel, patients, or visitors) Contaminated environmental surfaces
Contact-spread organisms (e.g., Staphylococcus aureus, drug-resistant gram-negative bacteria, Clostridium difficile, Group A Streptococcus infections, common respiratory viruses, or norovirus
Airborne infectious agents Serious, high-risk infection transmission breaches, with or without known infectious agents
Measles or tuberculosis contact investigations; might require large-scale notification and laboratory testing of contacts Patient notification, including considerations for bloodborne pathogen testing and prophylaxis
Contaminated water sources (e.g., sinks, ice machines, whirlpool bathtubs and hydrotherapy locations), aqueous medication preparation areas, or any device that generates mist Movement of patients across different healthcare facilities
Outbreaks of nontuberculosis mycobacterium skin and soft-tissue infections; Legionella; Pseudomonas species; Acinetobacter species; and other gram-negative bacteria Multifacility transmission of multidrug-resistant organisms across acute care, longterm care, dialysis, or outpatient settings
Emerging pathogens with potential for human-to-human transmission
Initial patient presentation for new influenza viruses, severe acute respiratory syndrome, Middle East respiratory syndrome, or viral hemorrhagic fever
Any setting where injections are administered
Contamination of injectable medications or solutions at point of manufacture, compounding pharmacy, or healthcare facility level
Outbreaks of different environmental organisms (e.g., gram-negative bacteria or fungi); syndromes might reflect mechanism of transmission (e.g., bloodstream infections after administration of contaminated intravenous medications, abscesses, or infections localized at the injection site)
Reuse of single-patient blood glucose monitoring devices on multiple patients
Outbreaks of bloodborne pathogens, especially hepatitis B or C virus, in long-term care settings
Diversion of narcotic drugs by healthcare personnel
Outbreaks of bloodborne pathogens, especially hepatitis C virus
Surgical and other invasive procedure settings (e.g., inpatient and outpatient surgical, podiatry, dental, ophthalmologic, plastic or cosmetic, or orthopedic centers)
Perioperative contamination of surgical wounds by healthcare personnel, the operative environment, or inadequately cleaned and sterilized instruments
Surgical site infections related to colonized healthcare workers (e.g., S. aureus, group A Streptococcus, Pseudomonas aeruginosa, nontuberculosis mycobacteria); contaminated devices (e.g., environmental bacteria or fungi), or other medical products (e.g., bandages or wound dressings); transmission of drug-resistant bacteria; adenovirus outbreaks in ophthalmology clinics and neonatal intensive care units; or nontuberculosis mycobacteria infections from contaminated heater– cooler units in open-chest surgeries
Endoscopy settings
Endoscope reprocessing errors or device design problems that prevent adequate cleaning and disinfection
Outbreaks of multidrug-resistant organisms associated with duodenoscopes; outbreaks of upper-and lower-respiratory tract infections associated with bronchoscopes; reported reprocessing errors and patient notification; pseudo-outbreaks of nontuberculosis mycobacteria
Table 18.5
Additional setting-specific outbreak and exposure scenarios
Transplant units
Dust exposure or air-handling problems for severely immunocompromised patient populations (e.g., during building construction or renovation)
Invasive mold infections in bone-marrow transplant units
Long-term care facilities
Group residential setting and limited infection control infrastructure or patients with high comorbidities
Outbreaks of multidrug-resistant bacteria, transmission across long-term care and acute care settings, respiratory viruses (especially seasonal influenza), or norovirus
Hemodialysis clinics
Lapses in injection safety, maintenance of dialysis machines, or central-line and other vascular access care
Bloodborne pathogens, especially hepatitis C virus and bloodstream infections
Dental clinics
Biofilm formation in inadequately maintained dental unit waterlines Inadequate cleaning and sterilization of dental surgical instruments
Outbreaks of nontuberculosis mycobacteria infections among children after pulpotomy procedures; or bloodborne pathogen exposures
Laboratory
Specimen collection, handling, or culture-related activities Contamination of microbiological specimens during collection, handling, or culture
Unintentional laboratory staff and other healthcare personnel exposures to bloodborne pathogens through needlesticks and splashes to mucous membranes; culture of tularemia or brucellosis; pseudo-outbreaks resulting in inappropriate invasive diagnostic procedures, antibiotic prescriptions, or extended hospitalizations
Conclusion
This chapter provided an overview of outbreak investigations of infections across various healthcare settings that may include acute care hospitals, urgent care centers, rehabilitation centers, nursing homes and other long-term care facilities, and specialized outpatient services (e.g., hemodialysis, dentistry, podiatry, chemotherapy, endoscopy, and pain management clinics). Investigations of outbreaks in these settings require special attention in comparison with traditional community outbreaks. In particular, investigations in healthcare settings may include other patients, healthcare personnel, medical devices, environmental surfaces, and environmental reservoirs (e.g., surfaces, air, and water). Additionally, healthcare investigations often require case finding, detailed line lists, infection control assessments, environmental sampling, implementing control measures, and patient notification. The chapter also emphasized coordination with the facility staff and leadership, as well as local and state public health when conducting the investigation. Investigating an outbreak in a healthcare setting requires a step-by-step process from verifying the diagnosis to identifying cases to notifying patients.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; the Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. https://www.cdc.gov/infection-control/hcp/isolation-precautions/
- Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare– associated infections. N Engl J Med. 2014;370:1198–208.
- CDC. Healthcare-associated infection (HAI) outbreak investigation toolkit. https://www.cdc.gov/healthcare-associated-infections/php/toolkit/outbreak-investigations-toolkit.html
- Blossom DB, Kallen AJ, Patel PR, Edward A, Robinson L. Outbreak of adverse reactions associated with contaminated heparin. N Engl J Med. 2008;359:2674–84.
- CDC. Infection control assessment tools. https://www.cdc.gov/healthcare-associated-infections/php/toolkit/icar.html
- CDC. Setting-specific guidelines. Guidelines library. https://www.cdc.gov/infectioncontrol/guidelines/index.html
- CDC. Interim Guidance for A Public Health Response to Contain Novel or Targeted Multidrug-Resistant Organisms (MDROs). Atlanta: US Department of Health and Human Services, CDC; [undated]. p. 1–9. https://www.cdc.gov/healthcare-associated-infections/media/pdfs/health-response-contain-mdro-h.pdf
- Institute of Medicine Forum on Microbial Threats. Summary and assessment. In: Global Infectious Disease Surveillance and Detection: Assessing the Challenges—Finding Solutions, Workshop Summary. Washington, DC: National Academies Press; 2007. http://www.ncbi.nlm.nih.gov/books/NBK52862
- CDC. Patient notification toolkit. https://www.cdc.gov/healthcare-associated-infections/hcp/patient-notification-toolkit/
- Patel PR, Srinivasan A, Perz JF. Developing a broader approach to management of infection control breaches in healthcare settings. Am J Infect Control. 2008;36:685–90.
- CDC. Outbreaks and patient notifications: resources for state health departments investigating healthcare-associated infection outbreaks and patient notifications. https://www.cdc.gov/healthcare-associated-infections/php/toolkit/outbreak-investigations-toolkit.html
