
Leishmaniasis
[Leishmania spp.]
Causal Agent
Leishmaniasis is a vector-borne disease that is transmitted by sandflies and caused by obligate intracellular protozoa of the genus Leishmania. Human infection is caused by about 21 of 30 species that infect mammals. These include the L. donovani complex with 3 species (L. donovani, L. infantum, and L. chagasi); the L. mexicana complex with 3 main species (L. mexicana, L. amazonensis, and L. venezuelensis); L. tropica; L. major; L. aethiopica; and the subgenus Viannia with 4 main species (L. (V.) braziliensis, L. (V.) guyanensis, L. (V.) panamensis, and L. (V.) peruviana). The different species are morphologically indistinguishable, but they can be differentiated by isoenzyme analysis, molecular methods, or monoclonal antibodies.
Life Cycle

Leishmaniasis is transmitted by the bite of infected female phlebotomine sandflies. The sandflies inject the infective stage (i.e., promastigotes) from their proboscis during blood meals  . Promastigotes that reach the puncture wound are phagocytized by macrophages
. Promastigotes that reach the puncture wound are phagocytized by macrophages  and other types of mononuclear phagocytic cells. Promastigotes transform in these cells into the tissue stage of the parasite (i.e., amastigotes)
and other types of mononuclear phagocytic cells. Promastigotes transform in these cells into the tissue stage of the parasite (i.e., amastigotes)  , which multiply by simple division and proceed to infect other mononuclear phagocytic cells
, which multiply by simple division and proceed to infect other mononuclear phagocytic cells  . Parasite, host, and other factors affect whether the infection becomes symptomatic and whether cutaneous or visceral leishmaniasis results. Sandflies become infected by ingesting infected cells during blood meals (
. Parasite, host, and other factors affect whether the infection becomes symptomatic and whether cutaneous or visceral leishmaniasis results. Sandflies become infected by ingesting infected cells during blood meals ( ,
,  ). In sandflies, amastigotes transform into promastigotes, develop in the gut
). In sandflies, amastigotes transform into promastigotes, develop in the gut  (in the hindgut for leishmanial organisms in the Viannia subgenus; in the midgut for organisms in the Leishmania subgenus), and migrate to the proboscis
(in the hindgut for leishmanial organisms in the Viannia subgenus; in the midgut for organisms in the Leishmania subgenus), and migrate to the proboscis  .
.
Geographic Distribution
Leishmaniasis is found in parts of about 88 countries. Approximately 350 million people live in these areas. Most of the affected countries are in the tropics and subtropics. The settings in which leishmaniasis is found range from rain forests in Central and South America to deserts in West Asia. More than 90 percent of the world’s cases of visceral leishmaniasis are in India, Bangladesh, Nepal, Sudan, and Brazil.
Leishmaniasis is found in Mexico, Central America, and South America—from northern Argentina to Texas (not in Uruguay, Chile, or Canada), southern Europe (leishmaniasis is not common in travelers to southern Europe), Asia (not Southeast Asia), the Middle East, and Africa (particularly East and North Africa, with some cases elsewhere).
Clinical Presentation
Human Leishmaniasis encompasses multiple clinical syndromes, most notably visceral, cutaneous, and mucosal forms. Infections can result in two main forms of disease, cutaneous leishmaniasis and visceral leishmaniasis (kala-azar). Different species can be associated with diverse clinical manifestations and sequelae. Species identification can facilitate clinical management, such as decisions regarding whether/which treatment is indicated. species, geographic location, and immune response of the host. Cutaneous leishmaniasis is characterized by one or more cutaneous lesions on areas where sandflies have fed. Persons who have cutaneous leishmaniasis have one or more sores on their skin. The sores can change in size and appearance over time. They often end up looking somewhat like a volcano, with a raised edge and central crater. A scab covers some sores. The sores can be painless or painful. Some people have swollen glands near the sores (for example, in the armpit if the sores are on the arm or hand).
Persons who have visceral leishmaniasis usually have fever, weight loss, and an enlarged spleen and liver (usually the spleen is bigger than the liver). Some patients have swollen glands. Certain blood tests are abnormal. For example, patients usually have low blood counts, including a low red blood cell count (anemia), low white blood cell count, and low platelet count. Some patients develop post kala-azar dermal leishmaniasis. Visceral leishmaniasis is becoming an important opportunistic infection in areas where it coexists with HIV.
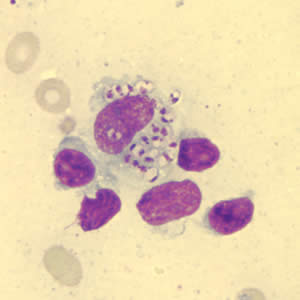
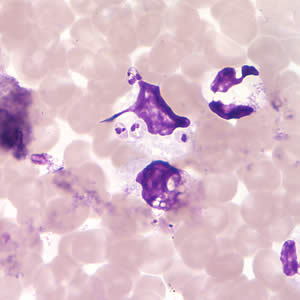
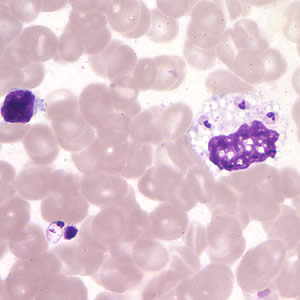
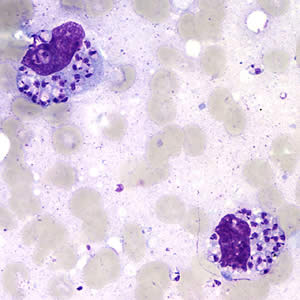
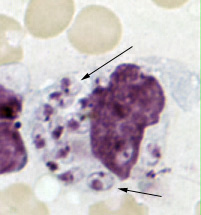
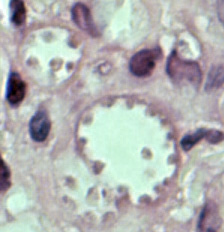
Leishmania amastigotes.
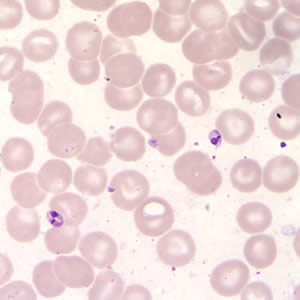
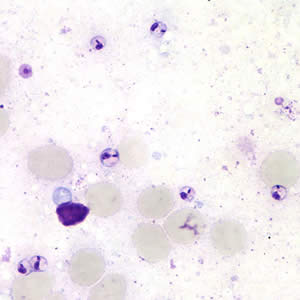
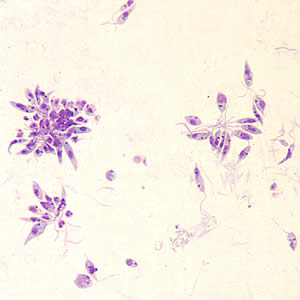
Leishmania sp. promastigotes from culture.
Diagnostic Findings
Microscopy
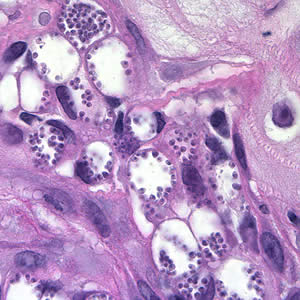
In the human host, only the amastigotes stage is seen upon microscopic examination of tissue specimens. Amastigotes can be visualized with both Giemsa and hematoxylin and eosin (H&E) stains. The amastigotes of Leishmania spp. are morphologically indistinguishable from those of Trypanosoma cruzi. Amastigotes are ovoid and measure 1-5 micrometers long by 1-2 micrometers wide. They possess both a nucleus and kinetoplast.
Isoenzyme analysis
Isolation can be done using the biphasic medium which includes a solid phase composed of blood agar base (e.g., NNN medium), with defribinated rabbit blood. After isolation parasites can be characterized to the complex and sometimes to the species level using isoenzyme analysis, which is the conventional diagnostic approach for Leishmania species identification. Diagnostic identification of Leishmania using this approach may take several weeks.
Serology
Antibody detection can prove useful in visceral leishmaniasis but is of limited value in cutaneous disease, since most patients do not develop a significant antibody response. In addition, cross reactivity can occur with Trypanosoma cruzi, a fact to consider when investigating Leishmania antibody response in patients who have been in Central or South America.
Molecular Diagnosis
Molecular approaches have the potential to be more sensitive and rapid; e.g., the results can be available within days versus weeks. CDC has incorporated molecular methods in the algorithm for the laboratory diagnosis of leishmaniasis. The method is based on PCR amplification using generic primers that amplify a segment of the rRNA internal transcribed spacer 2 (ITS2) from multiple Leishmania species. DNA sequencing analysis is performed on the amplified fragment for species identification. This approach allows the differentiation among Viannia spp., namely, L. (V.) braziliensis, L. (V.) guyanensis, and L. (V.) panamensis as well as L. (L.) aethiopica, L. (L.) amazonensis, L. (L.) donovani, L. (L.) infantum/chagasi, (L.) major, L. (L.) mexicana and L. L. (L.) tropica
Newly Released
“Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH) ”
Treatment Information
Treatment information for leishmaniasis can be found at: https://www.cdc.gov/leishmaniasis/hcp/clinical-care/index.html
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.