
Hymenolepiasis
[Hymenolepis diminuta] [Hymenolepis nana]
Causal Agents
Hymenolepiasis is caused by two cestodes (tapeworm) species, Hymenolepis nana (the dwarf tapeworm, adults measuring 15 to 40 mm in length) and Hymenolepis diminuta (rat tapeworm, adults measuring 20 to 60 cm in length). Hymenolepis diminuta is a cestode of rodents infrequently seen in humans and frequently found in rodents.
Life Cycles
Hymenolepis nana
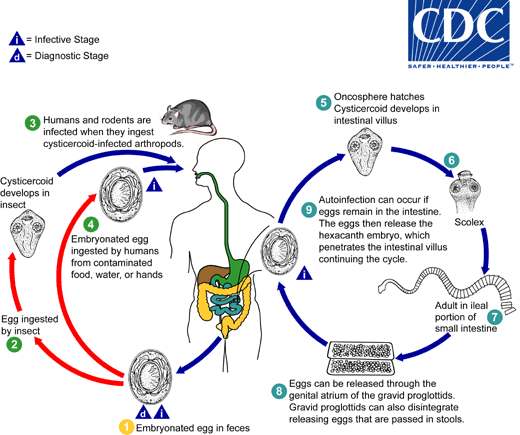
Eggs of Hymenolepis nana are immediately infective when passed with the stool and cannot survive more than 10 days in the external environment  . When eggs are ingested by an arthropod intermediate host
. When eggs are ingested by an arthropod intermediate host  (various species of beetles and fleas may serve as intermediate hosts), they develop into cysticercoids, which can infect humans or rodents upon ingestion
(various species of beetles and fleas may serve as intermediate hosts), they develop into cysticercoids, which can infect humans or rodents upon ingestion  and develop into adults in the small intestine. A morphologically identical variant, H. nana var. fraterna, infects rodents and uses arthropods as intermediate hosts. When eggs are ingested
and develop into adults in the small intestine. A morphologically identical variant, H. nana var. fraterna, infects rodents and uses arthropods as intermediate hosts. When eggs are ingested  (in contaminated food or water or from hands contaminated with feces), the oncospheres contained in the eggs are released. The oncospheres (hexacanth larvae) penetrate the intestinal villus and develop into cysticercoid larvae
(in contaminated food or water or from hands contaminated with feces), the oncospheres contained in the eggs are released. The oncospheres (hexacanth larvae) penetrate the intestinal villus and develop into cysticercoid larvae  . Upon rupture of the villus, the cysticercoids return to the intestinal lumen, evaginate their scoleces
. Upon rupture of the villus, the cysticercoids return to the intestinal lumen, evaginate their scoleces  , attach to the intestinal mucosa and develop into adults that reside in the ileal portion of the small intestine producing gravid proglottids
, attach to the intestinal mucosa and develop into adults that reside in the ileal portion of the small intestine producing gravid proglottids  . Eggs are passed in the stool when released from proglottids through its genital atrium or when proglottids disintegrate in the small intestine
. Eggs are passed in the stool when released from proglottids through its genital atrium or when proglottids disintegrate in the small intestine  . An alternate mode of infection consists of internal autoinfection, where the eggs release their hexacanth embryo, which penetrates the villus continuing the infective cycle without passage through the external environment
. An alternate mode of infection consists of internal autoinfection, where the eggs release their hexacanth embryo, which penetrates the villus continuing the infective cycle without passage through the external environment  . The life span of adult worms is 4 to 6 weeks, but internal autoinfection allows the infection to persist for years.
. The life span of adult worms is 4 to 6 weeks, but internal autoinfection allows the infection to persist for years.
Hymenolepis diminuta
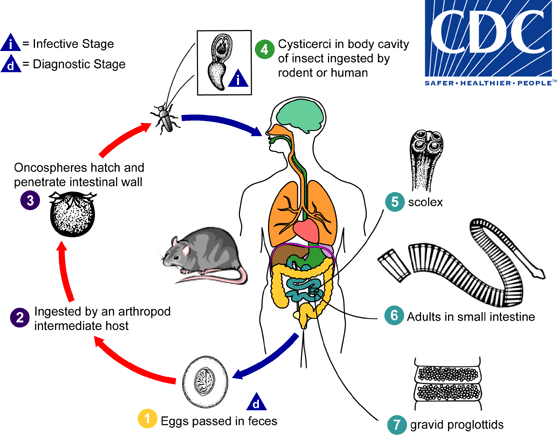
Eggs of Hymenolepis diminuta are passed out in the feces of the infected definitive host (rodents, man)  . The mature eggs are ingested by an intermediate host (various arthropod adults or larvae)
. The mature eggs are ingested by an intermediate host (various arthropod adults or larvae)  , and oncospheres are released from the eggs and penetrate the intestinal wall of the host
, and oncospheres are released from the eggs and penetrate the intestinal wall of the host  , which develop into cysticercoid larvae. Species from the genus Tribolium are common intermediate hosts for H. diminuta. The cysticercoid larvae persist through the arthropod’s morphogenesis to adulthood. H. diminuta infection is acquired by the mammalian host after ingestion of an intermediate host carrying the cysticercoid larvae
, which develop into cysticercoid larvae. Species from the genus Tribolium are common intermediate hosts for H. diminuta. The cysticercoid larvae persist through the arthropod’s morphogenesis to adulthood. H. diminuta infection is acquired by the mammalian host after ingestion of an intermediate host carrying the cysticercoid larvae  . Humans can be accidentally infected through the ingestion of insects in precooked cereals, or other food items, and directly from the environment (e.g., oral exploration of the environment by children). After ingestion, the tissue of the infected arthropod is digested releasing the cysticercoid larvae in the stomach and small intestine. Eversion of the scoleces
. Humans can be accidentally infected through the ingestion of insects in precooked cereals, or other food items, and directly from the environment (e.g., oral exploration of the environment by children). After ingestion, the tissue of the infected arthropod is digested releasing the cysticercoid larvae in the stomach and small intestine. Eversion of the scoleces  occurs shortly after the cysticercoid larvae are released. Using the four suckers on the scolex, the parasite attaches to the small intestine wall. Maturation of the parasites occurs within 20 days and the adult worms can reach an average of 30 cm in length
occurs shortly after the cysticercoid larvae are released. Using the four suckers on the scolex, the parasite attaches to the small intestine wall. Maturation of the parasites occurs within 20 days and the adult worms can reach an average of 30 cm in length  . Eggs are released in the small intestine from gravid proglottids
. Eggs are released in the small intestine from gravid proglottids  that disintegrate after breaking off from the adult worms. The eggs are expelled to the environment in the mammalian host’s feces
that disintegrate after breaking off from the adult worms. The eggs are expelled to the environment in the mammalian host’s feces  .
.
Geographic Distribution
Hymenolepis nana is the most common cause of all cestode infections, and is encountered worldwide. In temperate areas its incidence is higher in children and institutionalized groups. Hymenolepis diminuta, while less frequent, has been reported from various areas of the world.
Clinical Presentation
Hymenolepis nana and H. diminuta infections are most often asymptomatic. Heavy infections with H. nana can cause weakness, headaches, anorexia, abdominal pain, and diarrhea.
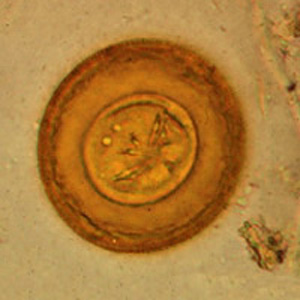
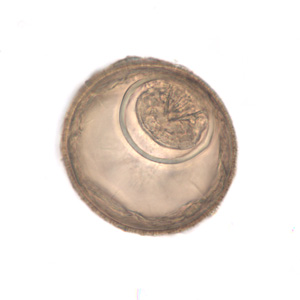
Hymenolepis diminuta eggs in wet mounts.
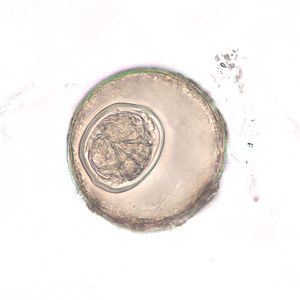
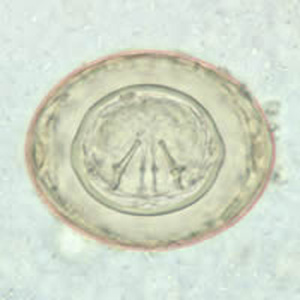
Hymenolepis nana egg in wet mounts.
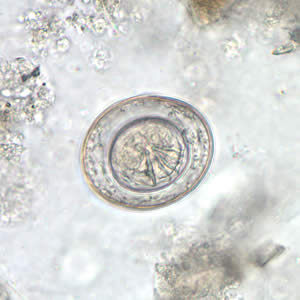
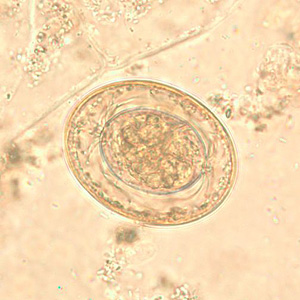
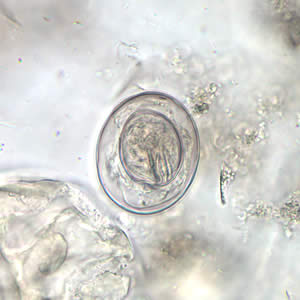
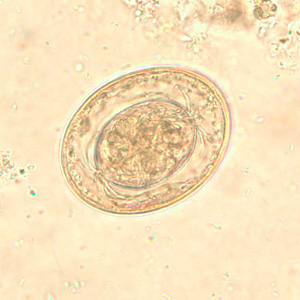
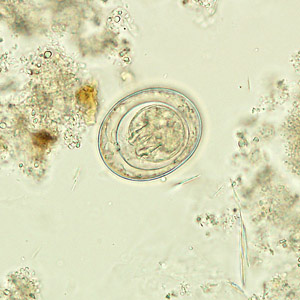
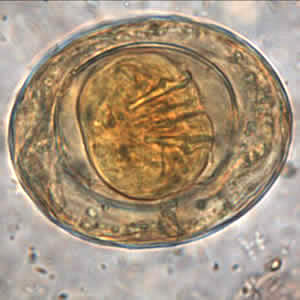
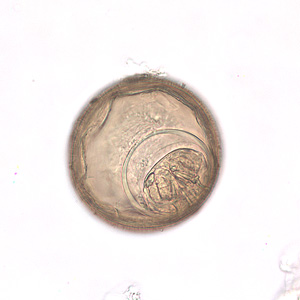
Hymenolepis nana eggs, zinc PVA trichrome stain.
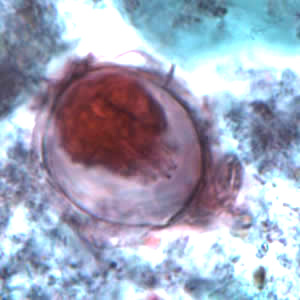
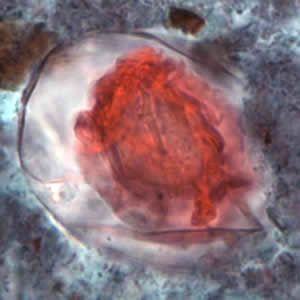
Hymenolepis proglottids.
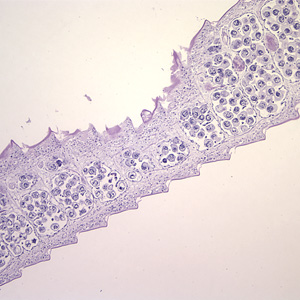
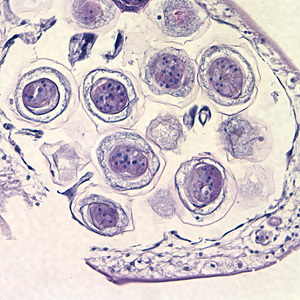
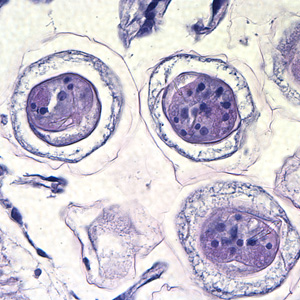

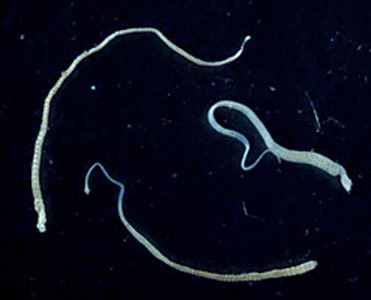
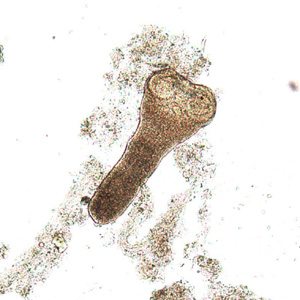
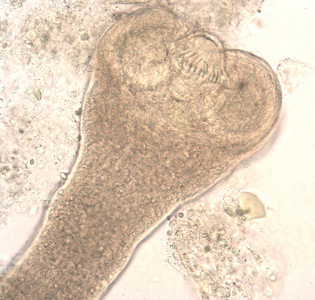
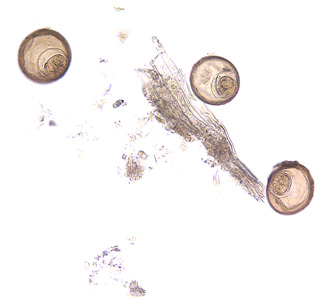
Intermediate hosts of Hymenolepis spp.


Diagnostic Findings
The diagnosis depends on the demonstration of eggs in stool specimens. Concentration techniques and repeated examinations will increase the likelihood of detecting light infections.
Treatment Information
Treatment information for hymenolepiasis can be found at: https://www.cdc.gov/hymenolepis/hcp/clinical-care/index.html
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.