Key points
- Clean and heat sterilize handpieces and other intraoral instruments that can be removed from the air- and waterlines of dental units.
- If a dental handpiece cannot be heat sterilized and does not have US Food and Drug Administration (FDA) clearance with validated instructions for reprocessing, do not use that device.
Why it matters
The internal components of air-driven dental handpieces (both low-speed and high-speed devices) can become contaminated with patient material during use.
If these devices are not properly cleaned and heat sterilized, the next patient may be exposed to potentially infectious materials.
Background
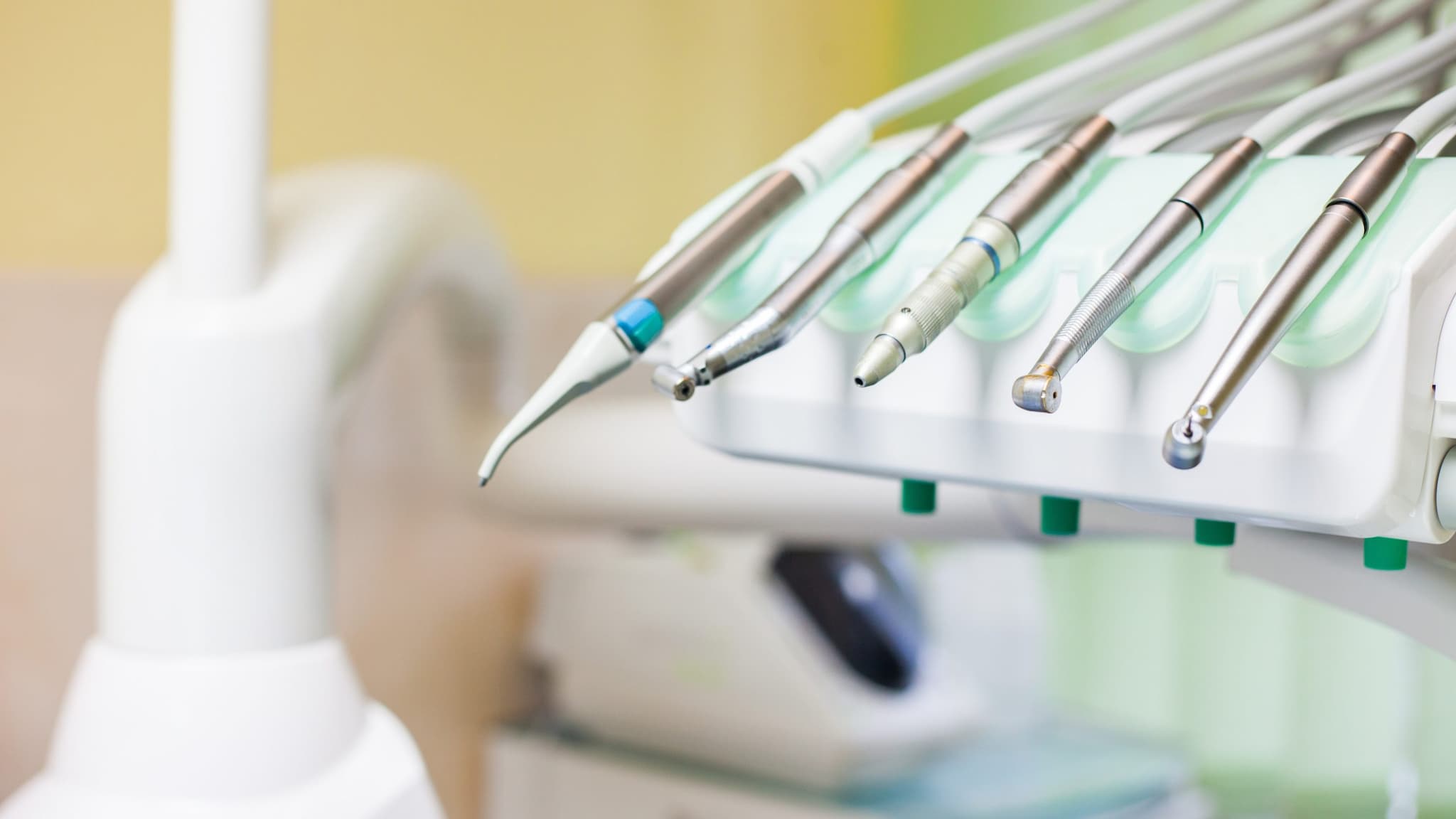
Dental handpieces include high-speed, low-speed, electric, endodontic, and surgical handpieces and all attachments, such as prophy angles and nose cones. These devices should always be heat sterilized between patients. For any device that can be removed from the dental unit air- or waterline, dental health care personnel (DHCP) should follow the validated manufacturer’s instructions for reprocessing these devices. These instructions for cleaning, lubrication, and sterilization ensure effectiveness of the process and longevity of handpieces.
Neither surface disinfection nor immersion in germicides are acceptable methods for reprocessing. Do not subject the handpiece to high-level disinfection and do not simply wipe the surface with a low-level disinfectant.
If a dental handpiece cannot be heat sterilized and does not have FDA clearance with validated instructions for reprocessing, DHCP should not use that device.
Recommendations
CDC recommends that DHCP use FDA-cleared devices and reprocess instruments between each patient according to the manufacturer’s instructions for use.
More information on dental handpieces can be found on pages 30–31 and 45 in Guidelines for Infection Control in Dental Health-Care Settings—2003.
Special considerations
Some handpieces are independent of air- and waterlines (e.g., cordless devices). For these items, CDC recommends that DHCP follow current FDA regulations. DHCP should utilize only FDA-cleared devices and follow the validated manufacturer's reprocessing instructions (cleaning, lubricating, and/or sterilizing).
DHCP should also follow the validated manufacturer's instructions for reprocessing handpiece couplers.
- Chin JR, Miller CH, Palenik CJ. Internal contamination of air-driven low-speed handpieces and attached prophy angles. J Am Dent Assoc. 2006;137:1275–1280.
- Chin JR, Westerman AE, Palenik CJ, Eckert SG. Contamination of handpieces during pulpotomy therapy on primary teeth. Pediatr Dent. 2009;31:71–75.
- Herd S, Chin J, Palenik CJ, Ofner S. The in vivo contamination of air-driven low-speed handpieces with prophylaxis angles. J Am Dent Assoc. 2007;138:1360–1365.
- Rutala WA, Weber DJ, and the Healthcare Infection Control Practices Advisory Committee. Guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2008. Accessed February 14, 2024. https://www.cdc.gov/infection-control/hcp/disinfection-and-sterilization/index.html
- US Food and Drug Administration. Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. Guidance for Industry and Food and Drug Administration Staff. US Dept of Health and Human Services; 2015. https://www.fda.gov/media/80265/download
