What to know
- COVID-19 vaccines help our bodies develop immunity to the virus that causes COVID-19 without us having to get the illness.
- Different COVID-19 vaccines may work in our bodies differently but all provide protection against the virus that causes COVID-19.
Overview
Different types of vaccines work in different ways to offer protection. But with all types of vaccines, the body is left with a supply of “memory” T-lymphocytes as well as B-lymphocytes that will remember how to fight that virus in the future.
It typically takes a few weeks after vaccination for the body to produce T-lymphocytes and B-lymphocytes.
Sometimes after vaccination, the process of building immunity can cause symptoms, such as fever. These symptoms are normal signs the body is building immunity.
Types of vaccines
Available Vaccines
There are different types of vaccines.
- All COVID-19 vaccines prompt our bodies to recognize and help protect us from the virus that causes COVID-19.
- Currently, there are two types of COVID-19 vaccines for use in the United States: mRNA, and protein subunit vaccines.
None of these vaccines can give you COVID-19.
- Vaccines do not use any live virus.
- Vaccines cannot cause infection with the virus that causes COVID-19 or other viruses.
COVID-19 vaccines do not affect or interact with our DNA.
- These vaccines do not enter the nucleus of the cell where our DNA (genetic material) is located, so they cannot change or influence our genes.
mRNA vaccines (Pfizer-BioNTech or Moderna)
To trigger an immune response, many vaccines put a weakened or inactivated germ into our bodies. Not mRNA vaccines. Instead, mRNA vaccines use mRNA created in a laboratory to teach our cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies. This immune response, which produces antibodies, is what helps protect us from getting sick from that germ in the future.
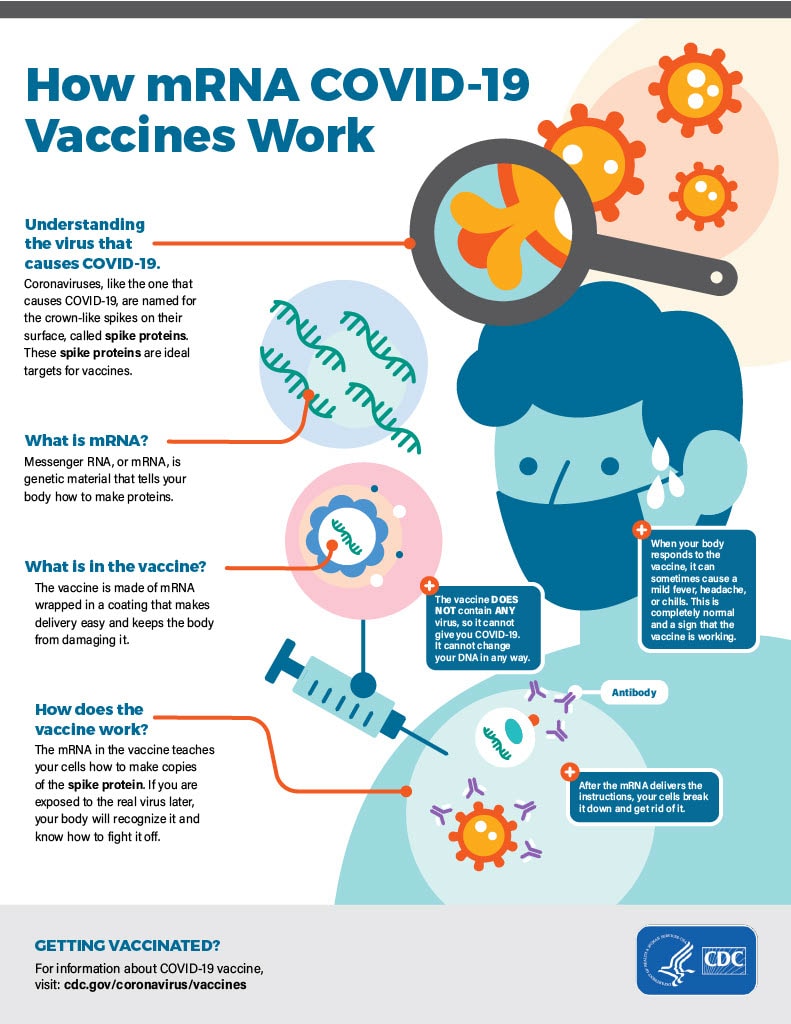
How mRNA COVID-19 vaccines work
- First, mRNA COVID-19 vaccines are given in the upper arm muscle or upper thigh, depending on the age of who is getting vaccinated.
- After vaccination, the mRNA will enter the muscle cells. Once inside, they use the cells’ machinery to produce a harmless piece of what is called the spike protein. The spike protein is found on the surface of the virus that causes COVID-19. After the protein piece is made, our cells break down the mRNA and remove it, leaving the body as waste.
- Next, our cells display the spike protein piece on their surface. Our immune system recognizes that the protein does not belong there. This triggers our immune system to produce antibodies and activate other immune cells to fight off what it thinks is an infection. This is what your body might do if you got sick with COVID-19.
- At the end of the process, our bodies have learned how to help protect against future infection with the virus that causes COVID-19. The benefit is that people get this protection from a vaccine, without ever having to risk the potentially serious consequences of getting sick with COVID-19. Any side effects from getting the vaccine are normal signs the body is building protection.
Research for mRNA technology
Researchers have been studying and working with mRNA vaccines for decades.
- In fact, mRNA vaccines have been studied before for flu, Zika, rabies, and cytomegalovirus (CMV).
- Beyond vaccines, cancer research has also used mRNA to trigger the immune system to target specific cancer cells.
Protein subunit vaccines (Novavax)
Protein subunit vaccines contain pieces (proteins) of the virus that causes COVID-19. These virus pieces are the spike protein. The vaccine also contains another ingredient called an adjuvant that helps the immune system respond to that spike protein in the future. Once the immune system knows how to respond to the spike protein, the immune system will be able to respond quickly to the actual virus spike protein and protect you against COVID-19.
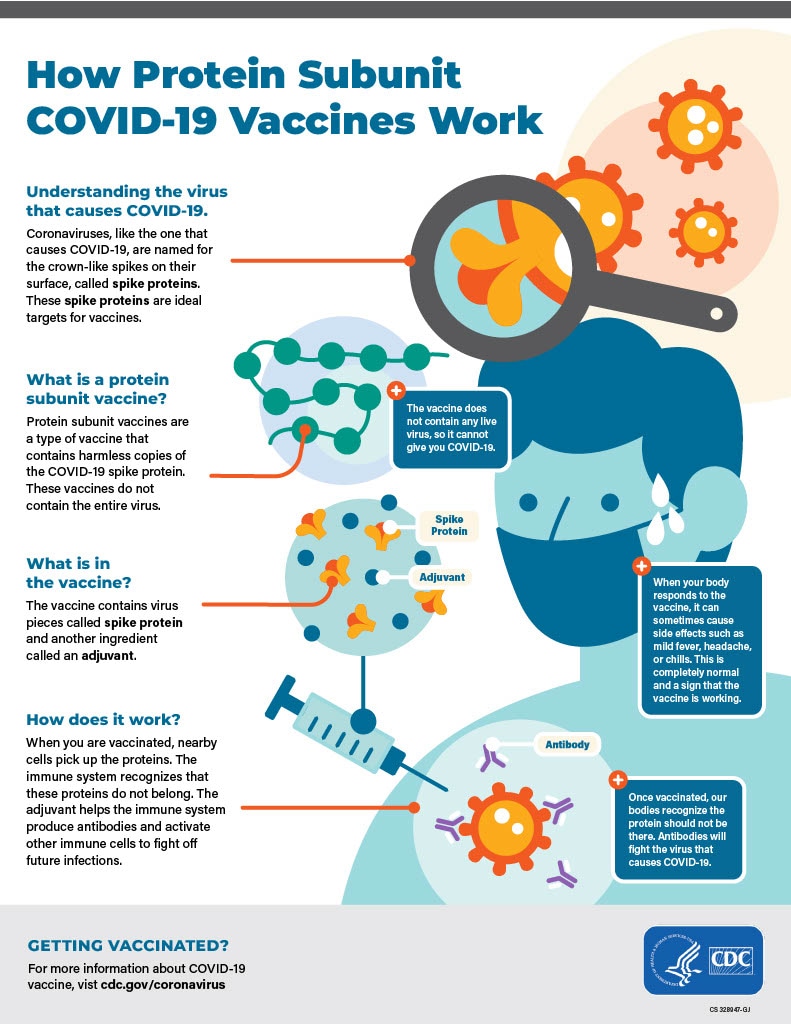
How protein subunit COVID-19 vaccines work
- Protein subunit COVID-19 vaccines are given in the upper arm muscle. After vaccination, nearby cells pick up these proteins.
- Next, our immune system recognizes that these proteins do not belong there. Another ingredient in the vaccine, the adjuvant, helps our immune system to produce antibodies and activate other immune cells to fight off what it thinks is an infection. This is what your body might do if you got sick with COVID-19.
- At the end of the process, our bodies have learned how to help protect against future infection with the virus that causes COVID-19. The benefit is that people get this protection from a vaccine, without ever having to risk the potentially serious consequences of getting sick with COVID-19. Many side effects from getting the vaccine are normal signs the body is building protection.
Research for protein subunit technology
Protein subunit vaccines have been used for years.
- More than 30 years ago, a hepatitis B vaccine became the first protein subunit vaccine to be approved for use in people in the United States.
- Another example of protein subunit vaccines used today include whooping cough vaccines.
About developing COVID-19 vaccines
Overview:
While COVID-19 vaccines were developed rapidly, all steps have been taken to ensure their safety and effectiveness. Bringing a new vaccine to the public involves many steps including:
- vaccine development,
- clinical trials,
- U.S. Food and Drug Administration (FDA) authorization or approval, and
- development and approval of vaccine recommendations through the Advisory Committee on Immunization Practices (ACIP) and CDC.
As vaccines are distributed outside of clinical trials, monitoring systems are used to make sure that COVID-19 vaccines are safe.
Initial Development
New vaccines are first developed in laboratories. Scientists have been working for many years to develop vaccines against coronaviruses, such as those that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). SARS-CoV-2, the virus that causes COVID-19, is related to these other coronaviruses. The knowledge that was gained through past research on coronavirus vaccines helped speed up the initial development of the current COVID-19 vaccines.
Clinical Trials
After initial laboratory development, vaccines go through three phases of clinical trials to make sure they are safe and effective. No trial phases have been skipped.
The clinical trials for COVID-19 vaccines have involved tens of thousands of volunteers of different ages, races, and ethnicities.
Clinical trials for vaccines compare outcomes (such as how many people get sick) between people who are vaccinated and people who are not. Results from these trials have shown that COVID-19 vaccines are safe and effective, especially against severe illness, hospitalization, and death.
Authorization or Approval
Before vaccines are made available to people in real-world settings, FDA assesses the findings from clinical trials. Initially, they determined that COVID-19 vaccines met FDA’s safety and effectiveness standards and granted those vaccines Emergency Use Authorizations (EUAs). The EUAs allowed the vaccines to be quickly distributed for use while maintaining the same high safety standards required for all vaccines. Learn more in this video about EUAs.
FDA has granted full approval for some COVID-19 vaccines. Before granting approval, FDA reviewed evidence that built on the data and information submitted to support the EUA. This included:
- preclinical and clinical trial data and information,
- details of the manufacturing process,
- vaccine testing results to ensure vaccine quality, and
- inspections of the sites where the vaccine is made.
These vaccines were found to meet the high standards for safety, effectiveness, and manufacturing quality FDA requires of an approved product. Learn more about the process for FDA approval.
Vaccine Recommendations
When FDA authorizes or approves a COVID-19 vaccine, ACIP reviews all available data about that vaccine to determine whether to recommend it and who should receive it. These vaccine recommendations then go through an approval process that involves both ACIP and CDC.
Tracking Safety Using Vaccine Monitoring Systems
Hundreds of millions of people in the United States have received COVID-19 vaccines under the most intense safety monitoring in U.S. history.
Several monitoring systems continue to track outcomes from COVID-19 vaccines to ensure their safety. Some people have no side effects. Many people have reported common side effects after COVID-19 vaccination, like pain or swelling at the injection site, a headache, chills, or fever. These reactions are common and are normal signs that your body is building protection.
Reports of serious adverse events after vaccination are rare.
