At a glance
This appendix summarizes recommendations for health care providers on management of intrauterine devices when users are found to have pelvic inflammatory disease. This information comes from the 2024 U.S. Selected Practice Recommendations for Contraceptive Use (U.S. SPR). The U.S. SPR provides recommendations for health care providers that address a selected group of common, yet sometimes complex, issues regarding initiation and use of specific contraceptive methods.
Figure F1.
Figure F1. Management of intrauterine devices when users of copper intrauterine devices or levonorgestrel intrauterine devices are found to have pelvic inflammatory disease*
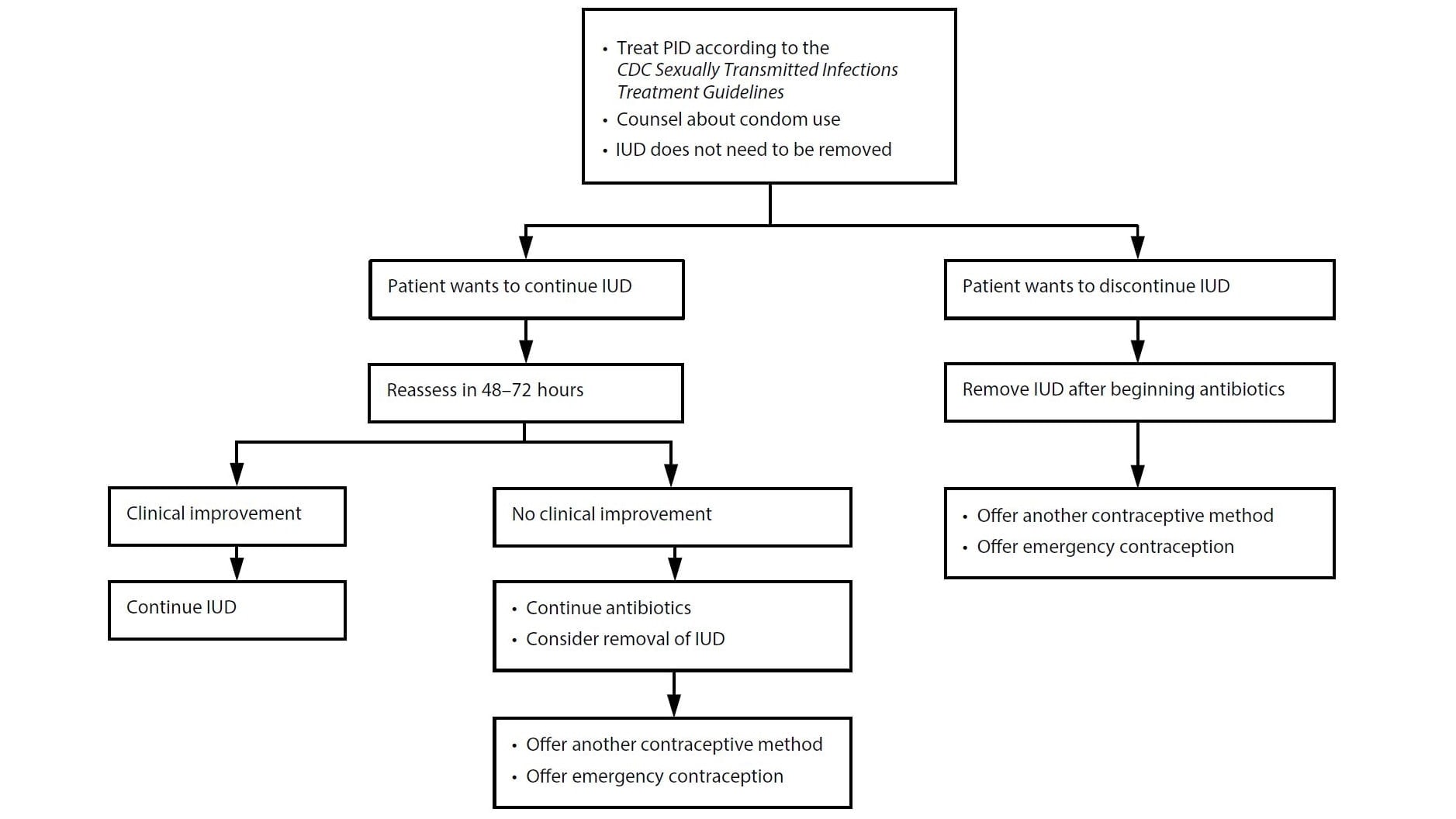
Abbreviations: IUD = intrauterine device; PID = pelvic inflammatory disease.
* Refer to CDC Sexually Transmitted Infections Treatment Guidelines for information on PID diagnostic considerations and treatment regimens.
