Key points
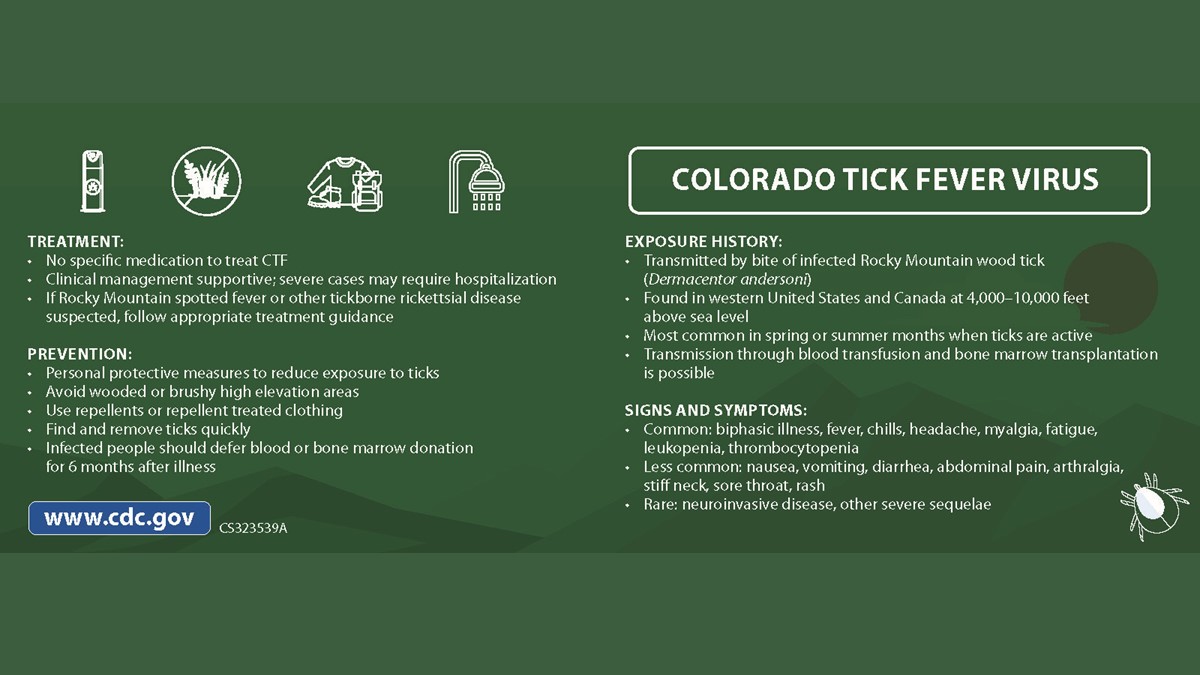
- Colorado tick fever (CTF) is usually a self-limited febrile illness with a biphasic course.
- Reverse transcription-polymerase chain reaction (RT-PCR) is the most sensitive test early in the course of CTF.
- Contact your state or local health department for assistance with diagnostic testing.

Clinical considerations
Preliminary diagnosis of CTF is based on the patient’s signs and symptoms, places and dates of travel, activities, and history of potential tick exposure.
CTF cases are reportable to local public health authorities in certain states. As of 2023, CTF was reportable in ten states: Arizona, Colorado, Idaho, Montana, New Mexico, Oregon, South Dakota, Utah, Washington, and Wyoming.
Signs and symptoms
CTF is a self-limited infection characterized by sudden onset of fever, chills, headache, myalgia, and malaise. Sore throat, vomiting, abdominal pain, and a maculopapular or petechial rash also have been reported. At least half of patients experience a biphasic illness, and adults >30 years of age may have prolonged weakness and fatigue.
More severe sequelae, including meningitis, encephalitis, hepatitis, epididymo-orchitis, pericarditis, myocarditis, pneumonia, and coma, can rarely occur. Death is also rare and usually associated with disseminated intravascular coagulation or meningoencephalitis in children.
Laboratory abnormalities observed in patients with CTF can be largely attributed to infection of hemopoietic progenitor cells. Leukopenia is commonly observed. Differential white blood cell counts may show a relative lymphocytosis and the presence of atypical lymphocytes. Moderate thrombocytopenia is also common.
Diagnostic testing
Laboratory diagnosis of CTF is generally accomplished by testing of serum to detect viral RNA or virus-specific immunoglobulin (Ig) M and neutralizing antibodies. Antibody production can be delayed with CTF, so tests that measure antibodies might not be positive for 14–21 days after the onset of symptoms. RT-PCR is a more sensitive test early in the course of disease.
CTF testing is available at some commercial and state health department laboratories and at CDC. Contact your state or local health department for assistance with diagnostic testing. They can help you determine if samples should be sent to the CDC Arbovirus Diagnostic Laboratory for further testing.
- Staples JE (2022). Reoviruses: Colorado tick fever virus and other vector-borne reoviruses. In: Kaslow RA, Stanberry LR, LeDuc JW (eds). Viral Infections of Humans. Springer, New York, NY. doi: 10.1007/978-1-4939-9544-8_62-1
- McDonald E, George D, Rekant S, et al. Notes from the Field: Investigation of Colorado tick fever virus disease cases — Oregon, 2018. Morb Mortal Wkly Rep. 2019;68(12):289–290. doi: 10.15585/mmwr.mm6812a4
- Yendell SJ, Fischer M, Staples JE. Colorado tick fever in the United States, 2002-2012. Vector Borne Zoonotic Dis. 2015;15(5):311–316. doi: 10.1089/vbz.2014.1755
- Lambert AJ, Kosoy O, Velez JO, Russell BJ, Lanciotti RS. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods. 2007;140(1-2):43–48. doi: 10.1016/j.jviromet.2006.10.014