At a glance
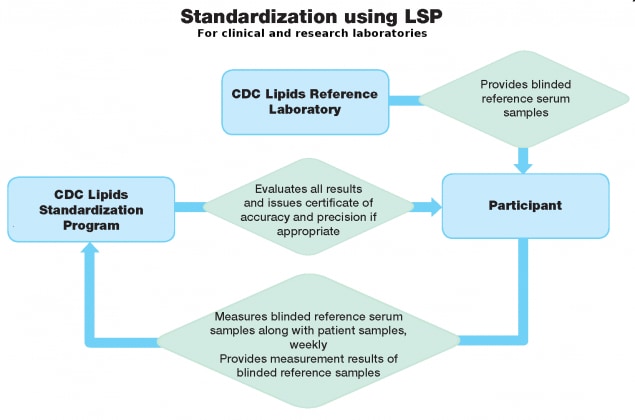
The Lipids Standardization Program (LSP) monitors the accuracy of research and clinical laboratories over time. LSP participants (clinical and research laboratories) report their measurement results from the LSP samples to CDC where results are evaluated.

Accuracy-based Monitoring Program (AMP) for CVD
Formerly known as Lipids Standardization Program (LSP)
The Lipids Standardization Program (LSP) monitors the accuracy of research and clinical laboratories over time. LSP ensures the analytical accuracy and precision of measurements performed in research studies and routine clinical laboratories by providing blinded standards (LSP samples) traceable to the CDC Reference Laboratory for measuring total cholesterol (TC), glycerides (TG), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A-I (apo A-I), and apolipoprotein B (apo B).
The LSP is unique among external quality-control systems (EQAS) because it provides a way to establish, assess, and improve the analytical accuracy and precision measurements over time. Other programs assess analytical measurement performance at a particular point in time without taking previous measurement results into account.
Keep in mind
LSP participants (clinical and research laboratories) report their measurement results from the LSP samples to CDC where results are evaluated. Those participants meeting defined performance criteria for accuracy and precision receive a certificate of performance and are considered CDC-certified.
AMP for CVD - LSP 101
What does LSP do?
LSP monitors the accuracy and precision of its laboratory participants over time by collecting weekly measurement results from samples provided to them. It uses statistical procedures to evaluate measurement accuracy and precision over a period of three months, and issues quarterly reports to its participants.
What do LSP participants do?
LSP participants analyze one sample provided to them weekly, for 12 weeks. Participants are unaware of each sample's biomarker concentrations. These samples must be included in routine analytical runs, along with clinical samples.
What happens when participants do not meet the performance criteria?
If performance criteria are not met, the participant should take appropriate corrective actions. Contact the program for additional guidance.
What are acceptable performance criteria in LSP?
Criteria for Acceptable Performance for the CDC Lipids Standardization Program (expressed in mg/dL)
| Analyte | Concentration Range | Maximum Allowable Bias | Maximum Allowable Standard Deviation |
|---|---|---|---|
| HDL-C | <40.0 ≥40.0 |
0.05 (RV) 0.05 (RV) |
1.7 0.04 (RV) |
| TC | 100-149.9 > 149.9 |
0.03 (RV) 0.03 (RV) |
4.0 0.03 |
| TG | 0.0-88.0 > 88-176.0 > 176.0-220.0 > 220 |
9 10 11 0.05 (RV) |
7 8 10 0.05 (RV) |
Criteria for Acceptable Performance for the CDC Lipids Standardization Program (expressed in mmol/L)
| Analyte | Concentration Range | Maximum Allowable Bias | Maximum Allowable Standard Deviation |
|---|---|---|---|
| HDL-C (mmol/L) | <1.03 ≥1.03 |
0.05 (RV) 0.05 (RV) |
0.0440 0.04 (RV) |
| TC (mmol/L) | 2.586-3.877 > 3.877 |
0.03 (RV) 0.03 (RV) |
0.103 0.03 (RV) |
| TG (mmol/L) | 0.00-0.994 > 0.994-1.989 > 1.989-2.486 > 2.486 |
0.102 0.113 0.124 0.05 (RV) |
0.079 0.090 0.113 0.05 (RV) |
Ready to enroll in LSP?
Please contact us at cdclsp@cdc.gov to request an application packet and submit the completed application for approval.
