At a glance
- A virus-like particle chikungunya vaccine (VIMKUNYA) is available in the United States.
- The vaccine is licensed for people aged 12 years and older.
- Vaccination should be considered for some travelers at higher risk of exposure to chikungunya virus and for some laboratory workers.

Overview
The virus-like particle vaccine (VIMKUNYA), manufactured by Bavarian Nordic, is currently licensed and approved for use in the United States. The vaccine was licensed by the Food and Drug Administration (FDA) in February 2025 and the Advisory Committee on Immunization Practices (ACIP) approved recommendations for use in U.S. travelers and laboratory workers in April 2025.
Recommendations for travelers
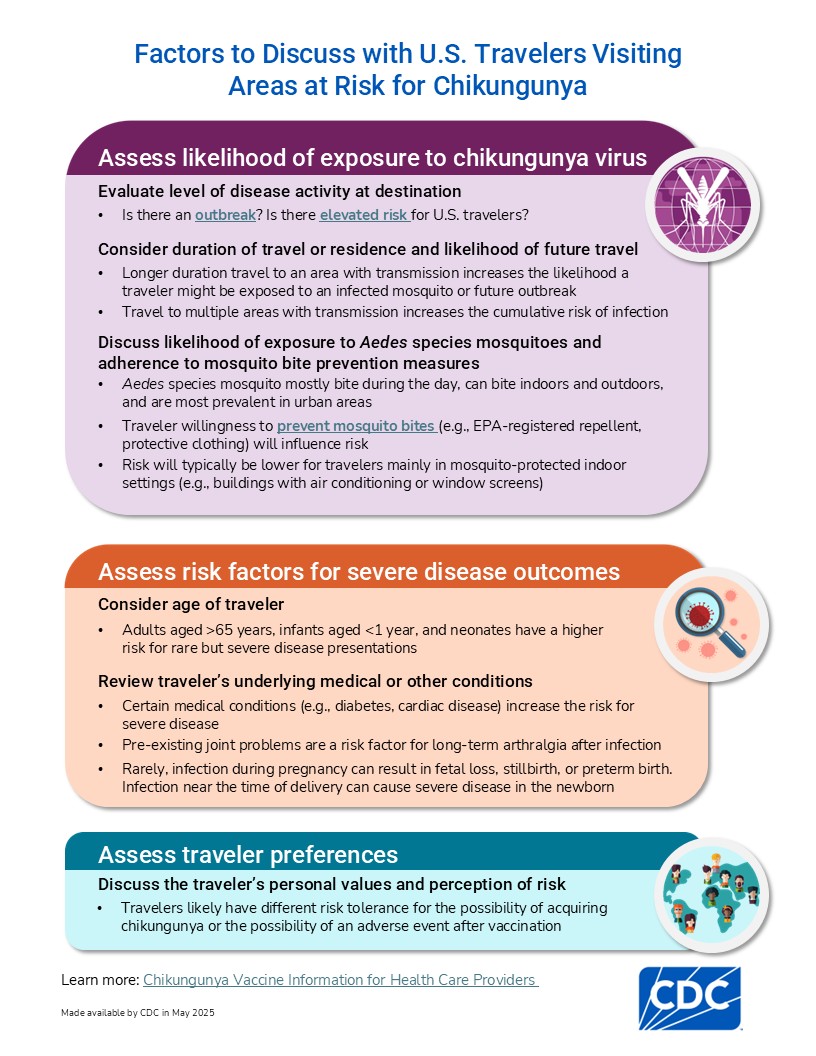
The risk of chikungunya for most U.S. travelers is low. However, some travelers are at higher risk for infection based on their destination or duration of travel. Healthcare providers can use CDC's decision tree to help determine when chikungunya vaccination for travelers is appropriate.
ACIP recommendations
Vaccine recommended
The vaccine is recommended for people aged 12 years and older traveling to a country or territory where a chikungunya outbreak is occurring.
Vaccine may be considered
The vaccine may be considered for people aged 12 years and older who are traveling or moving to a country or territory without an outbreak but with elevated risk for U.S. travelers if planning to stay for an extended period of time (e.g., 6 months or more).
Recommendations for laboratory workers
ACIP recommends chikungunya vaccine for laboratory workers with potential for exposure to chikungunya virus.
Laboratory work with chikungunya virus is generally restricted to Biosafety Level 3 (BSL-3) facilities and practices. A local institutional biosafety committee should undertake a risk assessment of the potential for exposure to chikungunya virus for each laboratory worker working with the virus. The committee should consider the type of work to be performed and biosafety level at which work will be conducted. Vaccination is not necessary for workers handling clinical samples who should routinely use standard practices for handling patient samples.
Precautions and contraindications
To minimize the risk of serious adverse events, healthcare providers should carefully observe contraindications and consider precautions for vaccination before vaccine administration.
Contraindications
- History of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine.
Precautions
Altered immune status
- Immunocompromised people, including those with an immunodeficiency or immunosuppression from disease or medications, might have a diminished immune response to the vaccine.
Pregnancy
- Pregnant women should avoid the risk for chikungunya virus infection, if possible.
- In general, vaccination should be deferred until after delivery. However, when the risk of infection is high (e.g., due to an outbreak) and exposure cannot be avoided, a healthcare provider and pregnant woman should discuss the risks of chikungunya virus infection and the potential benefits and risks of vaccination.
- If a pregnant woman chooses to be vaccinated, deferring vaccination until after the first trimester (after 14 weeks gestation) might be preferred.
- The vaccine should ideally be administered a minimum of 2 weeks before the expected date of delivery—preferably earlier—to allow for protection around the time of delivery.
Side effects of vaccination
Reporting adverse events
Common adverse reactions following vaccination that occurred in more than 10% of vaccinated participants aged 12–64 years in clinical trials included injection site pain, fatigue, headache, and myalgia. Common adverse reactions that occurred in more than 5% of vaccinated participants aged 65 years and older in clinical trials included injection site pain, fatigue, and myalgia.
Effectiveness and duration of protection
Clinical trial results indicated that vaccination resulted in seroresponse rates of 98% for people aged 12–64 years and 87% for people aged 65 years and older at 3 weeks after vaccination. At 6 months after vaccination, rates were 85% and 76% for the two age groups, respectively. Data on long-term seroresponse rates are still being gathered.
Administering the vaccine
- Administered intramuscularly
- Single 0.8mL dose
- Currently no recommendations for a booster dose

![[thumbnail] (hidden)](/chikungunya/media/images/2025/05/Chikungunya-vaccine_DecisionTree_Jan2026_p-508.jpg)