Key points
- To prevent harmful reactions, such as difficulty swallowing and trouble breathing, talk to your provider before getting botulinum toxin injections.
- Ask whether the product was purchased from an authorized source of FDA-approved botulinum toxin.
- Ask whether the provider has a valid healthcare license and is trained to give the injections.
- If in doubt, don't get the injections and seek another, properly licensed provider.
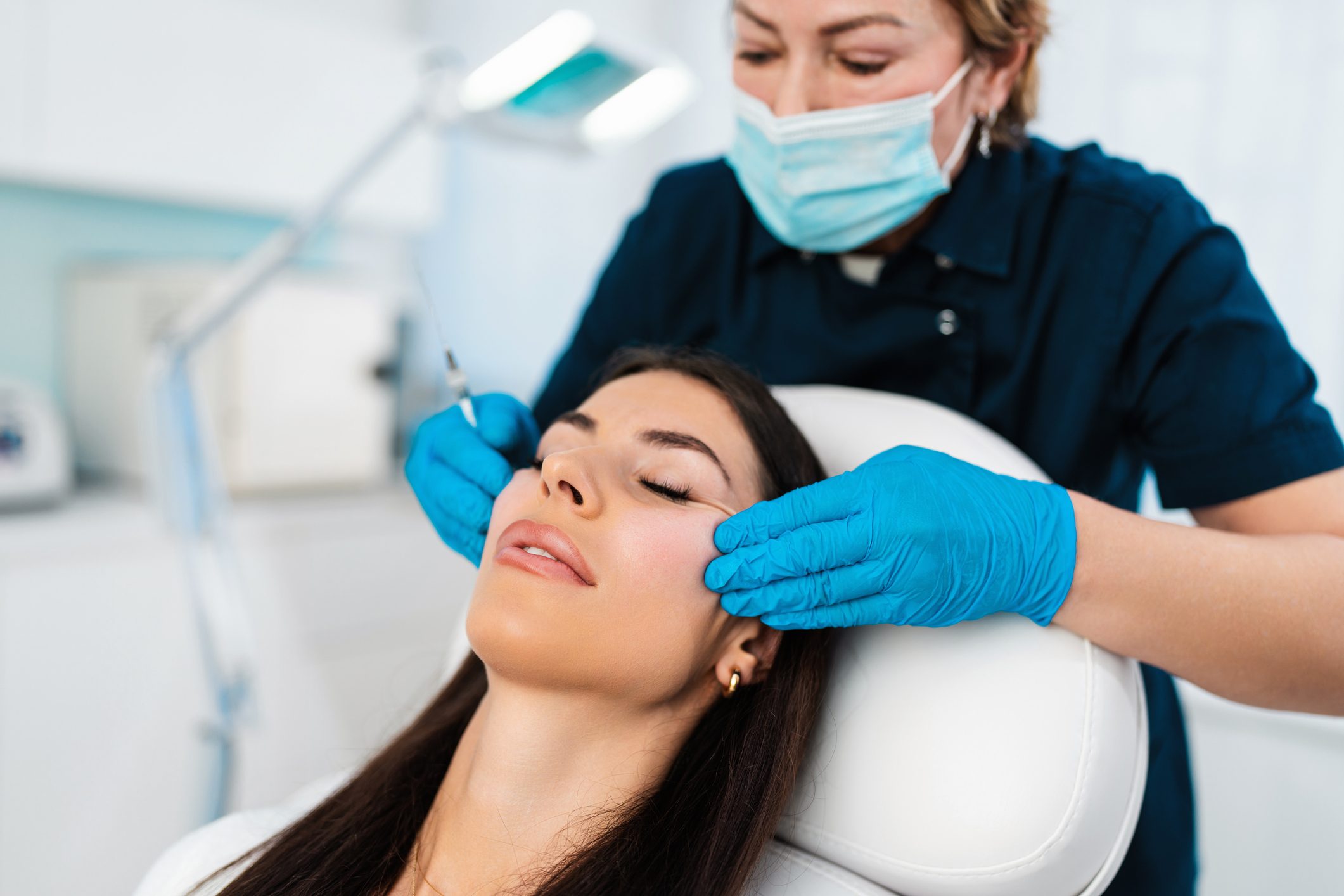
What is botulinum toxin?
For some of us, looking great means a wrinkle-free face.
Many things can help with preventing or smoothing wrinkles, including limiting exposure to direct sunlight, applying broad-spectrum sunscreen, using skin care products with retinoids, and getting certain cosmetic procedures.
A popular cosmetic procedure to reduce the appearance of wrinkles is the use of very low doses of botulinum toxin. When injected into facial muscles, botulinum toxin paralyzes the muscles, preventing them from tightening, so wrinkles don't show as much. The effect usually lasts several months.
Botulinum toxin is one of the most powerful toxins known to science, capable of causing life-threatening paralysis even in small doses. Cosmetic botulinum toxin products should be carefully prepared by their manufacturer and confirmed to have very small amounts of toxin. FDA approval of these products ensures they are safe when administered properly by qualified, licensed healthcare workers.
People commonly use "Botox®" as a name for all botulinum toxin products and procedures. Botox® is a registered trademark and the brand name for the first FDA-approved cosmetic botulinum toxin product. Other FDA-approved cosmetic botulinum toxin products include Daxxify®, Dysport®, Jeuveau®, Letybo®, and Xeomin®.
What you should know
Some people are being harmed
CDC has received an increased number of reports of harmful reactions, including difficulty swallowing and trouble breathing, after injection with botulinum toxin. Some people were hospitalized and some needed antitoxin, a treatment for botulism. Botulism is a rare but serious illness. It happens when botulinum toxin attacks the body's nerves and causes difficulty breathing, muscle paralysis, and even death.
What we know
- Some people with harmful reactions were injected with products purchased from unlicensed sources online.
- None of the products were FDA-approved; some were counterfeit; and some were unlabeled.
- Many of these products mimicked the appearance of safe, FDA-approved products.
- Some people received injections from providers who did not have a valid healthcare license or were not trained to safely inject botulinum toxin products.
- A valid healthcare license is required to purchase and inject botulinum toxin products.
- Some people injected themselves.
- Some people were injected in non-healthcare settings, including homes or spas.
- We don’t know what’s in unapproved, counterfeit, “knock off,” or “black market” botulinum toxin products. Products purchased from unlicensed sources could be dangerous, even life-threatening.
- They might contain harmful ingredients and might be misbranded, adulterated, counterfeit, contaminated, or improperly stored and transported.
- They also might contain an amount of botulinum toxin different from the amount on the product's carton or vial. Injection with too much toxin could lead to botulism, including trouble breathing, and even death.
What you should do
If you are considering injections of botulinum toxin, talk to your provider before getting the injections.
- Ask whether the botulinum toxin product is approved by FDA and was obtained from a licensed source.
- Ask whether the provider has a valid healthcare license and is trained to give the injections.
- Your state might have a look-up tool where you can check whether a provider or a setting (such as a clinic or spa), has the appropriate license.
- Your state might have a look-up tool where you can check whether a provider or a setting (such as a clinic or spa), has the appropriate license.
- If in doubt, don't get the injections and seek another, properly licensed provider.
For extra peace of mind,
- Ask the provider to show you the vial of FDA-approved botulinum toxin product.
- Ask the provider to draw the toxin from the vial into the needle while you watch.
After getting injections
Seek emergency help if you have symptoms of botulism, such as
- Blurry or double vision
- Drooping eyelids
- Difficulty swallowing
- Difficulty breathing
- Muscle weakness
These symptoms can start hours to weeks after getting injections.
Botulism is a medical emergency
Report problems to the FDA.
- Report harmful reactions related to the use of any medication, including suspected counterfeit medication, to FDA's MedWatch Safety Information and Adverse Event Reporting Program.
- Report suspected counterfeit products to FDA at 800-551-3989 or through FDA's form for reporting suspected criminal activity.
What healthcare providers should do
- Consider the possibility of adverse effects from botulinum toxin injection, including for cosmetic reasons, when patients present with signs and symptoms consistent with botulism.
- Be aware of symptom overlap between the presentation of localized adverse effects from injection of botulinum toxin and the early symptoms of botulism. To help distinguish early botulism symptoms from localized adverse effects:
- Assess for symmetry of cranial nerve palsies; symmetric cranial nerve palsies are expected with botulism.
- Assess for progression of cranial nerve palsies, possibly followed by a descending symmetric flaccid paralysis. These should raise suspicion for botulism.
- Assess for clinical features distant from the injection site(s).
- If botulism is suspected, call the health department immediately for consultation and antitoxin release. If no one answers, contact the CDC Botulism Consultation Service at 770-488-7100 (available 24/7).
- Refer to your state or local health department for guidance on reporting adverse effects.
- Counsel patients who report using or being interested in using botulinum toxin about the risks of adverse events.
- Encourage patients to receive injections only from a provider with a valid healthcare license and the training required to properly administer FDA-approved botulinum toxin products.
- Report harmful reactions related to the use of any medication, including suspected counterfeit medication, to FDA's MedWatch Safety Information and Adverse Event Reporting Program.
Resources
- CDC Health Advisory: Adverse effects linked to counterfeit or mishandled botulinum toxin injections
- CDC Investigation Notice: Harmful reactions linked to counterfeit "Botox" or mishandled botulinum toxin injections
- FDA Investigation Notice: Counterfeit version of Botox found in multiple states
- Provider Resource: Know your source: Protecting patients from unsafe drugs
