Infant Botulism: Information for Clinicians
Call ⇒

Infant Botulism Treatment and Prevention Program at the California Department of Public Health
Available 24/7

If you suspect your patient has botulism, call immediately. Do not wait for laboratory confirmation.

The on-call physician will provide a no-cost clinical consultation and release BabyBIG treatment if the patient’s clinical findings indicate infant botulism.

Call your state health department to report the suspected case.
Treat ⇒

Prepare and administer BabyBIG as soon as it is received. Do not wait for laboratory confirmation.
BabyBIG®, human antitoxin for the treatment of infant botulism.
Photo courtesy of the California Department of Public Health.
Test ⇒

Submit specimens for diagnostic testing through your state health department’s laboratory or CDC. All patients receiving BabyBIG must be tested.

Some laboratories, including CDC, do not routinely accept specimens unless BabyBIG has been released.

Questions about testing and reporting should be directed to your state health department.
Receive

The laboratory will report results and notify the state health department of positive results.

Testing can take several days. If you do not hear back within 5 days, contact your state health department.
Call

Infant Botulism Treatment and Prevention Program at the California Department of Public Health
Available 24/7

If you suspect your patient has botulism, call immediately. Do not wait for laboratory confirmation.

The on-call physician will provide a no-cost clinical consultation and release BabyBIG treatment if the patient’s clinical findings indicate infant botulism.

Call your state health department to report the suspected case.
⇓
Treat

Prepare and administer BabyBIG as soon as it is received. Do not wait for laboratory confirmation.

BabyBIG®, human antitoxin for the treatment of infant botulism.
Photo courtesy of the California Department of Public Health.
⇓
Test

Submit specimens for diagnostic testing through your state health department’s laboratory or CDC. All patients receiving BabyBIG must be tested.

Some laboratories, including CDC, do not routinely accept specimens unless BabyBIG has been released.

Questions about testing and reporting should be directed to your state health department.
⇓
Receive

The laboratory will report results and notify the state health department of positive results.

Testing can take several days. If you do not hear back within 5 days, contact your state health department.
What Is Infant Botulism?
Infant botulism is an intestinal toxemia. The disease results after spores of the bacterium Clostridium botulinum or related species are swallowed, temporarily colonize an infant’s large intestine, and produce botulinum neurotoxin. The neurotoxin binds to cholinergic nerve terminals and cleaves intracellular proteins necessary for acetylcholine release, resulting in bulbar palsies, hypotonia, and a symmetric, descending, flaccid paralysis.
Signs and Symptoms
Patients with infant botulism may present with some or all the following signs and symptoms:
- Constipation
- Poor feeding
- Ptosis
- Sluggish pupils
- Flattened facial expression
- Diminished suck and gag reflexes
- Weak and altered cry
- Respiratory difficulty and possibly respiratory arrest
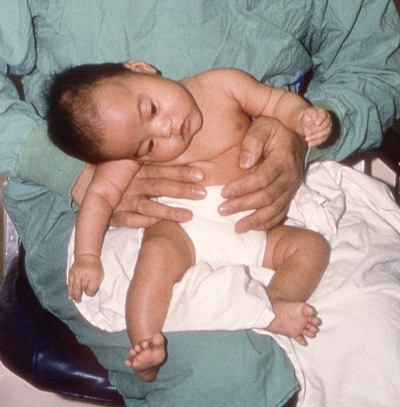
Patient recovering from infant botulism displaying continued neck muscle weakness with improved muscle tone, ptosis, and facial expression.
Photo courtesy of the California Department of Public Health.
Consultation
If the patient’s clinical findings are consistent with infant botulism, immediately contact the Infant Botulism Treatment and Prevention Program (IBTPP) at the California Department of Public Health for a free clinical consultation at 510-231-7600 (24/7).
After consulting with the IBTPP, call your state health department immediately to:
- Receive guidance on specimen collection, storage, and submission (also refer to the IBTPP collection guidance)
- Ask questions about testing methods
- Learn about mandatory reporting
After consulting with IBTPP, call your state health department. After-hours and emergency numbers can be found on CSTE’s website
Treatment
If clinical consultation supports botulism, request treatment immediately and administer it as soon as it’s available. Do not wait for laboratory confirmation.
Diagnostic Testing
A stool or enema specimen is required for definitive diagnosis of infant botulism. Enemas should be performed with sterile, non-bacteriostatic water. Stool specimens can be collected before or after antitoxin administration. Botulinum toxin can still be detected in the stool after BabyBIG administration because BabyBIG does not kill or prevent the growth of C. botulinum, inhibit the formation of botulinum toxin in the large intestine, or neutralize botulinum toxin present in the lumen of the intestine. Instead, BabyBIG neutralizes toxin present in blood, which can reduce complications and shorten hospital stay.
Diagnostic testing, including at CDC, is coordinated through the state health department’s laboratory. Follow up with your state health department if you do not receive test results within 5 days.
Notification and Reporting
Infant botulism is a notifiable disease in the United States.
Physicians must promptly notify the state health department of suspected cases, and laboratories must notify the state health department of all confirmed cases. As a courtesy, IBTPP notifies the state health department after BabyBIG infusion is completed.
State health departments report confirmed cases to CDC through the National Notifiable Diseases Surveillance System.
Who Do I Call?
To report suspected cases of infant botulism:
- Immediately contact the Infant Botulism Treatment and Prevention Program (IBTPP) at the California Department of Public Health for a free clinical consultation at 510-231-7600 (24/7). The IBTPP is available for additional consultation and information-sharing with state and local health departments 24/7, as needed.
- After speaking to the IBTPP, immediately contact your state health department to receive information on collecting, storing, and submitting specimens for analysis to the state public health laboratory or CDC. CDC accepts specimens for analysis from state public health laboratories and other federal agencies. Private healthcare providers and institutions may submit specimens directly to CDC with authorization from their state health department.
To report suspected cases of other kinds of botulism:
- Immediately contact your state health department.
- If no one answers, contact CDC’s Clinical Emergency Botulism Service at 770-488-7100 (24/7).
This page was created with assistance from the Infant Botulism Treatment and Prevention Program at the California Department of Public Health.
