Key points
- Initial diagnosis of infant botulism is based on clinical symptoms.
- Consultation is available for suspected cases.
- If clinical consultation supports infant botulism, begin treatment as soon as possible. Do not wait for laboratory confirmation.
- Infant botulism is a notifiable disease. All suspected cases must be reported to the state public health department.
Overview
Infant botulism is an intestinal toxemia.
The disease results after spores of the bacterium Clostridium botulinum or related species are swallowed. These spores temporarily colonize an infant's large intestine and produce botulinum neurotoxin.
The neurotoxin binds to cholinergic nerve terminals and cleaves intracellular proteins necessary for acetylcholine release. This can result in:
- Bulbar palsies
- Hypotonia
- A symmetric, descending, flaccid paralysis
Signs and symptoms
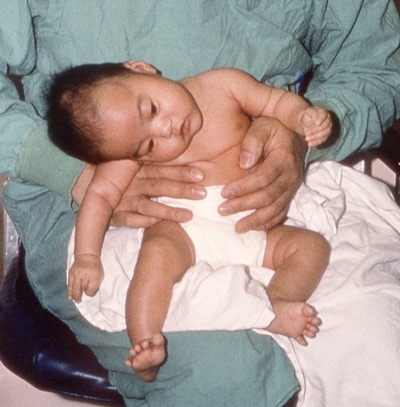
Patients with infant botulism may present with some or all the following signs and symptoms:
- Constipation
- Poor feeding
- Ptosis (drooping eyelid)
- Sluggish pupils
- Flattened facial expression
- Diminished suck and gag reflexes
- Weak and altered cry
- Respiratory difficulty and possibly respiratory arrest
Steps to take
Case consultation
If you suspect your infant patient has botulism, immediately call 510-231-7600 for case consultation. Consultation is available 24/7.
- You will reach the on-call physician at the Infant Botulism Treatment and Prevention Program (IBTPP) at the California Department of Public Health.
- The physician will provide a no-cost clinical consultation and release BabyBIG treatment if the patient's clinical findings indicate infant botulism.
Case reporting
After consulting with the IBTPP, call your state health department to:
- Receive guidance on specimen collection, storage, and submission (also refer to the IBTPP collection guidance)
- Ask questions about testing methods
- Learn about mandatory reporting
If clinical consultation supports infant botulism, begin treatment with BabyBIG as soon as it is available.
Keep in mind, initial diagnosis is based on clinical symptoms. Do not wait for laboratory confirmation to begin treatment.

A stool or enema specimen is required for definitive diagnosis of infant botulism. Enemas should be performed with sterile, non-bacteriostatic water.
Diagnostic testing is done through your state public health department laboratory. CDC provides testing services for some state public health departments.
This specialized testing often takes days to complete. Follow up with your state health department if you do not receive results within five days.
Infant botulism is a notifiable disease in the United States.
Physicians must promptly notify the state health department of suspected cases. As a courtesy, IBTPP notifies the state health department after BabyBIG infusion is completed. Laboratories must notify the state health department of all confirmed cases.
State health departments report confirmed cases to CDC through the National Notifiable Diseases Surveillance System.
- Photos are courtesy of the California Department of Public Health.
