At a glance
CDC published the Advisory Committee on Immunization Practices (ACIP) recommendation in 2014 for the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) among adults aged ≥65 years. CDC analyzed claims for vaccination submitted for reimbursements as a proxy for estimating vaccination coverage among adults aged ≥65 years before and after.
Authors: Carla L. Black, PhD; Walter W. Williams, MD; Rob Warnock; Tamara Pilishvili, PhD; David Kim, MD; Jeffrey A. Kelman, MD
Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; Acumen, LLC, Burlingame, California; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC; Center for Medicare, Centers for Medicare & Medicaid Services.
Introduction
On September 19, 2014, CDC published the Advisory Committee on Immunization Practices (ACIP) recommendation for the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) among adults aged ≥65 years, to be used in series with 23-valent pneumococcal polysaccharide vaccine (PPSV23)1. This replaced the previous recommendation that adults aged ≥65 years should be vaccinated with a single dose of PPSV23. As a proxy for estimating PCV13 and PPSV23 vaccination coverage among adults aged ≥65 years before and after implementation of these revised recommendations, CDC analyzed claims for vaccination submitted for reimbursement to the Centers for Medicare & Medicaid Services (CMS). The report provides proportions of claims for PPSV23 and PCV13 from any time during a beneficiary's enrollment in Medicare Parts A (hospital insurance) and B (medical insurance) since reaching age 65 years among beneficiaries continuously enrolled in Medicare Parts A and B during annual periods from September 19, 2009 through September 18, 2017.
Results
Claims for receipt of ≥1 dose of any pneumococcal vaccination (either PCV13 or PPSV23) among Medicare beneficiaries aged ≥65 years ranged from 40.0% by September 2010 to 59.3% by September 2017 (Figure 1). In that same period, claims for receipt of ≥1 dose of PPSV23 ranged from 40.0% to 44.5%. In the period following the ACIP recommendation for routine use of PCV13 in series with PPSV23 for adults aged ≥65 years, claims for receipt of ≥1 dose of PCV13 among Medicare beneficiaries aged ≥65 ranged from 14.8% by September 2015 to 40.0% by September 2017. Claims for receipt of both vaccines (i.e., at least 1 dose each of PCV13 and PPSV23) ranged from 8.7% by September 2015 to 23.8% by September 2017.
FIGURE 1. Proportion of Medicare beneficiaries aged ≥65 years with claims submitted for pneumococcal vaccination — United States, September 2009–September 2017*
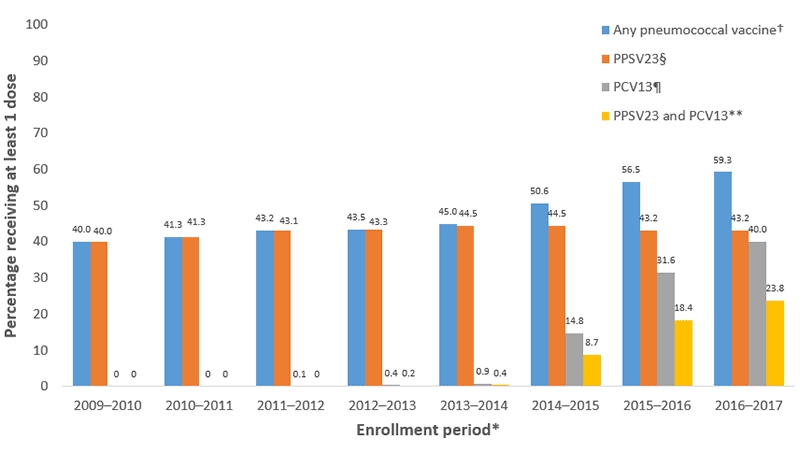
*Each enrollment period extends from September 19 of the first year through September 18 of the subsequent year, with the exception of the 2011-12 period, which ends on October 12, 2012, corresponding to the date of publication of the first recommendation for the use of 13-valent pneumococcal conjugate vaccine (PCV13) in series with 23-valent pneumococcal polysaccharide vaccine (PPSV23) in adults with certain immunocompromising conditions; denominators include all beneficiaries continuously enrolled in Medicare Parts A and B for the duration of the enrollment period.
†Percentage with at least one claim for PPSV23 or PCV13 since January 1, 1999 through the end of the enrollment period.
§Percentage with at least one claim for PPSV23 since January 1, 1999 through the end of the enrollment period.
¶ Percentage with at least one claim for PCV13 since January 1, 1999 through the end of the enrollment period.
** Percentage with at least one claim for PPSV23 and at least one claim for PCV13 since January 1, 1999 through the end of the enrollment period.
Claims for pneumococcal vaccination by September 2017 varied by demographic characteristics and the presence of chronic and immunocompromising medical conditions for which pneumococcal vaccination is recommended (Table 1). The percentages of beneficiaries with claims for all pneumococcal vaccine types were lowest among beneficiaries aged 65–69 years and highest among beneficiaries aged 80–84 years. Claims for PPSV23, PCV13, or both vaccines were higher among white beneficiaries than among beneficiaries of other racial/ethnic groups; the largest differences were between white and Hispanic beneficiaries (44.6% compared with 31.9% for PPSV23; 41.8% compared with 20.4% for PCV13; and 25.3% compared with 10.2% for both PPSV23 and PCV13). The percentages of beneficiaries aged ≥65 years with chronic medical conditions with claims for PPSV23 (47.2%), PCV13 (42.4%), and both vaccines (25.9%) were higher than for beneficiaries without these conditions (22.2%, 27.4%, and 12.9%, respectively). Similarly, the percentages of beneficiaries with immunocompromising medical conditions with claims for PPSV23, PCV13, and both vaccines were higher than the percentage among beneficiaries without these conditions (50.8% compared with 29.5%, 44.6% compared with 31.7%, and 28.2% compared with 16.0%, respectively).
TABLE 1. Proportion of Medicare beneficiaries aged ≥65 years with claims submitted for pneumococcal vaccination, by age, race/ethnicity, and presence of chronic and immunocompromising medical conditions, United States, September 2017*
| Category | Total enrolled beneficiaries | ≥1 dose PPSV23† | ≥1 dose PCV13§ | Both PPSV23 and PCV13¶ | Any pneumococcal** |
|---|---|---|---|---|---|
| Age (years) | |||||
| 65–69 | 8,139,239 | 25.1 | 35.0 | 14.7 | 45.4 |
| 70–74 | 6,178,432 | 42.8 | 42.1 | 25.0 | 59.9 |
| 75–79 | 4,508,374 | 53.6 | 43.9 | 30.3 | 67.3 |
| 80–84 | 3,127,193 | 59.5 | 43.4 | 31.8 | 71.1 |
| ≥85 | 3,263,056 | 58.9 | 39.6 | 28.1 | 70.4 |
| Race/ethnicity†† | |||||
| White | 21,540,349 | 44.6 | 41.8 | 25.3 | 61.2 |
| Black | 1,843,337 | 33.0 | 26.0 | 14.1 | 45.0 |
| Asian | 487,754 | 42.1 | 31.5 | 17.9 | 55.8 |
| Hispanic | 387,899 | 31.9 | 20.4 | 10.2 | 42.2 |
| American Indian/Alaskan Native | 116,949 | 36.3 | 32.9 | 15.7 | 53.5 |
| Other race | 435,741 | 40.0 | 35.8 | 20.7 | 55.2 |
| Immunocompromising condition§§ | |||||
| Yes | 16,192,237 | 50.8 | 44.6 | 28.2 | 67.2 |
| No | 9,024,057 | 29.5 | 31.7 | 16.0 | 45.2 |
| Chronic medical condition¶¶ | |||||
| Yes | 21,182,675 | 47.2 | 42.4 | 25.9 | 63.6 |
| No | 4,033,619 | 22.2 | 27.4 | 12.9 | 36.7 |
*Denominator in each subgroup includes all beneficiaries continuously enrolled in Medicare Parts A and B from September 19, 2016 through September 18, 2017.
† Percentage of beneficiaries with at least one claim for 23-valent pneumococcal polysaccharide vaccine (PPSV23) since January 1, 1999 through September 18, 2017.
§ Percentage of beneficiaries with at least one claim for 13-valent pneumococcal conjugate vaccine (PCV13) since January 1, 1999 through September 18, 2017.
¶ Percentage of beneficiaries with at least one claim for PPSV23 and at least one claim for PCV13 since January 1, 1999 through September 18, 2017.
** Percentage of beneficiaries with at least one claim for PPSV23 or PCV13 since January 1, 1999 through September 18, 2017.
†† Race/ethnicity was categorized as Hispanic or Latino, black, white, Asian, American Indian/Alaskan Native, and "other." Beneficiaries identified as Hispanic or Latino might be of any race. Beneficiaries identified as black, white, Asian, American Indian/Alaskan Native or other race are non-Hispanic. "Other" includes persons of multiple race. Excludes 3,263,056 beneficiaries with unknown race/ethnicity.
§ Includes cerebrospinal fluid leak, cochlear implant, sickle cell disease or other hemaglobinopathy, congenital or acquired asplenia, congenital or acquired immunodeficiency (including B- or T-lymphocyte deficiency, complement deficiencies, and phagocytic disorders excluding chronic granulomatous disease), HIV infection, chronic renal failure, nephrotic syndrome, leukemia, lymphoma, Hodgkin disease, generalized malignancy, immunosuppression due to treatment with immunosuppressive drugs, including long-term corticosteroids and radiation therapy, solid organ transplant, and multiple myeloma. Use of ICD codes might be nonspecific in identifying generalized malignancies if providers use these codes for "rule-out" diagnoses.
¶¶ Includes all immunocompromising conditions listed above plus chronic heart disease (including congestive heart failure and cardiomyopathies, excluding hypertension), chronic lung disease (including chronic obstructive pulmonary disease, emphysema, and asthma) and diabetes mellitus.
Claims for pneumococcal vaccination by September 2017 among beneficiaries aged ≥65 years also varied by state of residence (Table 2). Claims for PCV13 ranged from 29.7% in Mississippi to 61.8% in Wisconsin, claims for PPSV23 ranged from 26.2% in Alaska to 53.3% in Wisconsin, and claims for both vaccines ranged from 14.2% in Alaska to 41.6% in Wisconsin.
TABLE 2. Proportion of Medicare beneficiaries aged ≥65 years with claims submitted for pneumococcal vaccination, by state, United States, September 2017*
| State | Total enrolled beneficiaries | ≥1 dose PPSV23† | ≥1 dose PCV13§ | Both PPSV23 and PCV13¶ | Any pneumococcal** |
|---|---|---|---|---|---|
| Alabama | 396,887 | 41.0 | 30.3 | 17.9 | 53.5 |
| Alaska | 62,350 | 26.2 | 31.5 | 14.2 | 43.6 |
| Arizona | 521,340 | 41.0 | 37.8 | 21.2 | 57.6 |
| Arkansas | 319,131 | 42.7 | 35.8 | 21.9 | 56.6 |
| California | 2,153,280 | 40.2 | 34.5 | 19.7 | 55.0 |
| Colorado | 345,359 | 44.0 | 45.8 | 26.9 | 62.9 |
| Connecticut | 318,600 | 45.8 | 44.4 | 26.4 | 63.7 |
| Delaware | 124,055 | 48.9 | 49.4 | 30.4 | 67.9 |
| District of Columbia | 44,150 | 38.3 | 33.4 | 19.2 | 52.5 |
| Florida | 1,727,136 | 43.6 | 34.2 | 20.7 | 57.1 |
| Georgia | 678,159 | 42.7 | 39.0 | 23.2 | 58.5 |
| Hawaii | 86,839 | 41.2 | 40.5 | 24.2 | 57.4 |
| Idaho | 139,505 | 37.8 | 34.1 | 19.2 | 52.7 |
| Illinois | 1,114,427 | 43.6 | 40.1 | 24.7 | 59.0 |
| Indiana | 598,345 | 47.5 | 43.5 | 27.3 | 63.7 |
| Iowa | 359,239 | 45.2 | 49.8 | 30.5 | 64.6 |
| Kansas | 305,684 | 42.0 | 41.1 | 24.6 | 58.6 |
| Kentucky | 392,732 | 42.3 | 35.6 | 21.4 | 56.5 |
| Louisiana | 349,096 | 41.9 | 30.5 | 18.1 | 54.3 |
| Maine | 147,199 | 41.6 | 47.0 | 27.2 | 61.4 |
| Maryland | 591,451 | 44.4 | 44.4 | 26.4 | 62.4 |
| Massachusetts | 632,806 | 42.3 | 51.6 | 27.0 | 66.9 |
| Michigan | 824,537 | 45.1 | 37.9 | 23.4 | 59.6 |
| Minnesota | 224,294 | 50.2 | 56.6 | 37.1 | 69.7 |
| Mississippi | 317,146 | 38.8 | 29.7 | 17.0 | 51.5 |
| Missouri | 525,036 | 43.2 | 40.5 | 25.0 | 58.7 |
| Montana | 121,063 | 38.9 | 43.6 | 25.1 | 57.4 |
| Nebraska | 210,265 | 45.0 | 44.6 | 28.1 | 61.5 |
| Nevada | 203,022 | 34.0 | 31.0 | 16.5 | 48.6 |
| New Hampshire | 171,582 | 46.7 | 55.1 | 32.7 | 69.0 |
| New Jersey | 837,466 | 43.2 | 35.9 | 21.2 | 57.9 |
| New Mexico | 168,151 | 39.6 | 32.3 | 18.3 | 53.6 |
| New York | 1,336,233 | 42.2 | 36.5 | 22.0 | 56.6 |
| North Carolina | 852,687 | 47.0 | 47.3 | 29.5 | 64.8 |
| North Dakota | 71,479 | 44.4 | 49.5 | 31.0 | 62.9 |
| Ohio | 900,831 | 44.6 | 41.8 | 25.2 | 61.1 |
| Oklahoma | 389,646 | 43.7 | 35.9 | 21.0 | 58.5 |
| Oregon | 284,500 | 40.9 | 42.1 | 24.6 | 58.4 |
| Pennsylvania | 1,001,931 | 45.5 | 49.7 | 29.8 | 65.3 |
| Rhode Island | 72,118 | 36.6 | 40.7 | 19.7 | 57.5 |
| South Carolina | 516,479 | 42.7 | 42.0 | 24.0 | 60.7 |
| South Dakota | 92,534 | 42.7 | 44.9 | 27.8 | 59.8 |
| Tennessee | 547,244 | 44.1 | 41.3 | 24.6 | 60.8 |
| Texas | 1,673,303 | 43.8 | 33.1 | 19.9 | 56.9 |
| Utah | 158,496 | 41.6 | 42.4 | 22.5 | 61.4 |
| Vermont | 88,326 | 38.9 | 46.0 | 24.7 | 60.2 |
| Virginia | 803,159 | 47.0 | 48.6 | 29.8 | 65.7 |
| Washington | 589,455 | 40.6 | 42.9 | 24.8 | 58.7 |
| West Virginia | 188,326 | 38.1 | 30.2 | 17.0 | 51.3 |
| Wisconsin | 444,469 | 53.3 | 61.8 | 41.6 | 73.5 |
| Wyoming | 72,637 | 35.3 | 35.7 | 19.0 | 52.1 |
| Median | -- | 42.7 | 41.1 | 24.6 | 58.7 |
| Range across states | -- | 26.2–53.3 | 29.7–61.8 | 14.2–41.6 | 43.6–73.5 |
*Denominator in each subgroup includes all beneficiaries continuously enrolled in Medicare Parts A and B from September 19, 2016 through September 18, 2017.
† Percentage of beneficiaries with at least one claim for 23-valent pneumococcal polysaccharide vaccine (PPSV23) since January 1, 1999 through September 18, 2017.
§ Percentage of beneficiaries with at least one claim for 13-valent pneumococcal conjugate vaccine (PCV13) since January 1, 1999 through September 18, 2017.
¶ Percentage of beneficiaries with at least one claim for PPSV23 and at least one claim for PCV13 since January 1, 1999 through September 18, 2017.
** Percentage of beneficiaries with at least one claim for PPSV23 or PCV13 since January 1, 1999 through September 18, 2017.
Cumulative monthly claims for PCV13 among beneficiaries aged ≥65 years after publication of the September 2014 recommendation increased from 1.0% in September 2014 to 40.1% in September 2017 (Figure 2). The steepest monthly increase (3.7 percentage points) occurred from September 2015 to October 2015. During January–September 2017, the average monthly increase was 0.38 percentage points.
FIGURE 2. Percentage of Medicare beneficiaries aged ≥65 years with claims submitted for 13-valent pneumococcal conjugate vaccine (PCV13), by month*— United States, September 2014–September 2017†

*Percentage of beneficiaries with at least one claim for PCV13 before the end of the month of interest. Denominator each month includes beneficiaries continuously enrolled in Medicare Parts A and B for at least 12 months before and including the month of interest.
† The Advisory Committee on Immunization Practices recommendation for the routine use of PCV13 for adults aged ≥65 years was published September 19, 2014.
Discussion
Although the incidence of invasive pneumococcal disease (IPD) among adults aged ≥65 years has been dramatically reduced due to indirect effects of PCV13 use in children, in 2013 an estimated 13,500 cases of IPD occurred among adults aged ≥65 years, of which 20%–25% were caused by PCV13 serotypes12. Given that an additional 38% of IPD among adults aged ≥65 years is caused by serotypes unique to PPSV23, use of PCV13 and PPSV23 in series is expected to provide broader protection against IPD in older adults than the use of a single pneumococcal vaccine type (2). PCV13 has also demonstrated efficacy in preventing nonbacteremic pneumococcal pneumonia in adults aged ≥65 years3. In 2014, when ACIP recommended routine use of PCV13 in series with PPSV23 among adults aged ≥65 years, the addition of PCV13 was estimated to prevent 230 cases of IPD and approximately 12,000 cases of community-acquired pneumonia over the lifetime of a single cohort of persons aged 65 years in the United States1. Three years after the ACIP recommendation for routine use of PCV13 in series with PPSV23 in adults aged ≥65 years, claims for PCV13 reached 40% in September 2017. However, the expected benefits of PCV13 use in terms of cases of IPD and pneumonia prevented were estimated in a setting of 60% coverage immediately after vaccine introduction when there still remained a sizable vaccine-preventable disease burden4. Claims for PPSV23 were also persistently low, despite a long-standing recommendation for PPSV23 use in this population5.
The steepest monthly increase in PCV13 uptake coincided with the beginning of the 2015–16 influenza season. While less pronounced, monthly increases at the beginning of the 2016–17 influenza season were also greater than in the preceding months, suggesting that older adults might be receiving pneumococcal vaccination when they receive influenza vaccination, as has been previously reported6. Visits for influenza vaccination represent opportunities for providers to ensure that patients are up-to-date with other recommended vaccines. Implementation of the National Vaccine Advisory Committee's standards for adult immunization practice, which state that health care providers should assess vaccination status at every patient visit, recommend needed vaccines, and offer vaccination or refer to a vaccination service provider7, could help improve the initiation and completion of the pneumococcal vaccination series among adults aged ≥65 years to reduce the burden of pneumococcal pneumonia and IPD among these persons. In a recent survey of primary care physicians' knowledge and practices regarding ACIP-recommended pneumococcal vaccination for adults, >95% of respondents reported routinely assessing for and recommending both pneumococcal vaccines8. However, numerous barriers to vaccinating patients were identified, the most common being difficulty determining a patient's pneumococcal vaccination history. Consistent documentation of vaccine administration into state or local immunization information systems, another component of the standards for adult immunization practice, could help overcome this barrier.
Implementation of PCV13 and PPSV23 vaccination recommendations has not been equal across all adults aged ≥65 years. White beneficiaries were more likely to have claims for either type of vaccine than beneficiaries of other racial/ethnic groups, especially Hispanics and blacks. Differences in coverage with pneumococcal vaccine, as well as other vaccines, among older adults by race/ethnicity are well documented and might be attributable to differences in attitudes toward vaccination and concerns about vaccination safety, in provider recommendation for vaccination, and in quality of care received by different racial/ethnic groups910. Although PCV13 and PPSV23 are now routinely recommended for all adults aged ≥65 years, beneficiaries aged ≥65 years with chronic or immunocompromising medical conditions were more likely to have been vaccinated with both vaccines than those without such conditions. This higher percentage might be attributable to several factors: beneficiaries with chronic or immunocompromising medical conditions having more frequent provider contacts, and thus more opportunities for vaccination; providers being more aware of vaccination needs for persons with complicated medical conditions; and patients with chronic or immunocompromising conditions being more aware of the need for pneumococcal vaccination. Vaccination with both types of pneumococcal vaccine also varied by state, as has been previously reported for PPSV2310. State variation in vaccination coverage has been attributed to differences in health care delivery infrastructure and vaccination programs, as well as differences in population characteristics between states10.
The ACIP is currently reviewing the recommendation for routine use of PCV13 in adults aged ≥65 years and will revise as needed1. Long-term public health benefits of the recommendation could be limited due to continued indirect effects of PCV13 vaccination in children. Estimates of PCV13 vaccination coverage in adults aged ≥65 years are necessary to measure direct vaccine impact in the prevention of pneumococcal pneumonia and IPD, an important factor in the consideration of the revision of the recommendation for continued routine use of PCV13.
Methods
CDC monitored PCV13 and PPSV23 claims submitted for reimbursement to CMS among beneficiaries aged ≥65 years who were continuously enrolled in Medicare Parts A and BA during annual periods from September 19, 2009 through September 18, 2017. Enrollment periods covered the 5 years before through 3 years after the recommendation for routine use of PCV13 and PPSV23 in series for adults aged ≥65 years1. The number of beneficiaries per annual enrollment period ranged from 23.7 million to 25.2 million during these years. Beneficiaries were considered to be vaccinated with either PPSV23 or PCV13 or both if a claim for vaccination was submitted at any time during a beneficiary's history of enrollment in Medicare Parts A and B since reaching age 65 years and before the end of the enrollment period of interest. However, claims are only available in the CMS database beginning January 1, 1999. PCV13 and PPSV23 were identified by current procedural technology codes 90670 and 90732, respectively. Claims submitted from any hospital or outpatient setting (including pharmacies) were included. Claims submitted to CMS for at least 1 PCV13 dose (regardless of PPSV23 status), at least 1 PPSV23 dose (regardless of PCV13 status), at least 1 dose each of PCV13 and PPSV23, and at least 1 dose of either vaccine were stratified by age, race/ethnicity, state of residence, and the presence of chronic or immunocompromising medical conditions for which PCV13 or PPSV23 or both are indicated among adults aged <65 years11. Race/ethnicity was categorized as Hispanic or Latino, black, white, Asian, American Indian/Alaskan Native, and "other."B Chronic and immunocompromising medical conditions were identified by the presence of International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes listed on any claim submitted to CMS during a beneficiary's history of enrollment in Medicare Parts A and B through the end of the enrollment period of interest. The proportion of beneficiaries with claims submitted for PCV13 by the end of each month during September 2014–September 2017 was also assessed. The denominator for each month included beneficiaries continuously enrolled in Medicare Parts A and B for at least 12 months before and including the month of interest.
Limitations
The findings in this report are subject to at least five limitations related to the use of Medicare claims data as a proxy for estimating vaccination coverage. First, the percentage of beneficiaries in this study population with claims for pneumococcal vaccination might not be representative of pneumococcal vaccination coverage among all adults aged ≥65 years in the United States. The percentage with claims for any pneumococcal vaccine in this study (59.3%) differs from the 66.9% reported coverage with any pneumococcal vaccine among adults aged ≥65 years from the nationally representative 2016 National Health Interview Survey12. Second, exclusion from the current analysis of beneficiaries enrolled in Medicare Part C (Medicare is not billed separately for vaccinations for beneficiaries enrolled in Part C health plans) might have contributed to over- or underrepresentation of vaccinated persons in the study population. In 2017, 34% of Medicare beneficiaries were enrolled in Part C, with enrollment by state ranging from 2% to 59%13C. Third, the CMS database does not include claims for vaccinations administered before 1999. Whereas not having information on pneumococcal vaccination claims before 1999 would not affect estimates for PCV13 vaccination, the percentage of persons vaccinated with PPSV23 could be underestimated, particularly among older beneficiaries who reached age 65 years before 1999 and might have been vaccinated with PPSV23 after its licensure for use in the United States in 1983. Fourth, doses administered during hospitalization might not be captured if claims for the hospital stay were bundled. Finally, race/ethnicity of Hispanic beneficiaries and those of races other than white or black could potentially be misclassified because of the change in categorization of race/ethnicity information collected by the Social Security Administration in 198014.
- Analysis includes only Medicare beneficiaries in fee-for-service plans (Medicare Parts A and B). Beneficiaries receiving Medicare services through Medicare Part C (private plans that are paid a capitated rate for the basic Medicare services, also known as Medicare Advantage) are excluded. Under Part C health plans, Medicare is not billed separately for vaccinations.
- Beneficiaries identified as Hispanic or Latino might be of any race. Beneficiaries identified as black, white, Asian, American Indian/Alaskan Native, or other race are non-Hispanic. "Other" includes persons of multiple race. Race/ethnicity information for Medicare beneficiaries was historically obtained from the Social Security Administration's master beneficiary record. Before 1980, the Social Security application form only allowed classification of race into White, Black, and Other. Since 1980, the categories have been expanded to White (non-Hispanic); Black (non-Hispanic); Hispanic; Asian, Asian American, or Pacific Islander; American Indian or Alaska Native; and Unknown. The Health Care Financing Administration (now Centers for Medicare & Medicaid Services) conducted surveys of beneficiaries in attempts to better classify race/ethnicity of those enrolled before 1980. However, misclassification of race/ethnicity among beneficiaries included in the current analysis might remain, particularly among those of Hispanic ethnicity and races other than white or black.
- Although data are not available regarding differences in vaccination coverage between beneficiaries enrolled in Parts A and B versus Part C, if differences do exist, some of the variation in claims rates by state could be attributable to the large variation in the percentage of beneficiaries enrolled in Part C across states. The national estimate based on beneficiaries enrolled in Parts A and B could be biased even if coverage among Part C beneficiaries was similar, if residents of states with higher coverage are underrepresented because of a higher percentage of enrollment in Part C compared with residents of states with lower vaccination coverage.
- Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822–5.
- Pilishvili T and Bennett NM. Pneumococcal disease prevention among adults: strategies for the use of pneumococcal vaccines. Am J Prev Med 2015; 49(6S4):S383–S390.
- Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25.
- Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med 2016;31:901–8.
- Update: pneumococcal polysaccharide vaccine usage—United States. MMWR Morb Mortal Wkly Rep 1984;33:273–6, 281.
- Shen AK, Warnock R, Chu S, Kelman JA. Receipt of other routinely recommended vaccines relative to receipt of seasonal influenza vaccines: Trends from Medicare administrative data, 2013–2015. Vaccine 2018;36:4399-403.
- National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep 2014;129:115–23.
- Hurley LP, Allison MA, Pilishvili T, et al. Primary care physician's struggle with current adult pneumococcal vaccine recommendations. J Am Board Fam Med 2018;31:94–104.
- Lu PJ, O'Halloran A, Williams WW, Lindley MC, Farrall S, Bridges CB. Racial and ethnic disparities in vaccination coverage among adult populations in the U.S. Vaccine 2015;33(Suppl 4):D83–91.
- O'Halloran AC, Lu PJ, Pilishvili T. Pneumococcal vaccination coverage among persons ≥65 years—United States, 2013. Vaccine 2015;33:5503–6.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
- Hung MC, Williams WW, Lu PJ. Vaccination coverage among adults in the United States, National Health Interview Survey, 2016. AdultVaxView 2018. Available at https://www.cdc.gov/adultvaxview/publications-resources/vaccination-coverage-adults-2016.html.
- Centers for Medicare & Medicaid Services. Medicare enrollment dashboard. Baltimore, MD: Centers for Medicare & Medicaid Services; 2018. Available at: https://data.cms.gov/summary-statistics-on-beneficiary-enrollment/medicare-and-medicaid-reports/medicare-monthly-enrollment
- Eicheldinger C, Bonito A. More accurate racial and ethnic codes for Medicare administrative data. Health Care Financ Rev 2008;29:27–42.
