About
CDC vaccine recommendations are developed using an explicit evidence-based method based on the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Summary
On August 13, 2021, the Food & Drug Administration (FDA) approved an inactivated TBE vaccine (manufactured by Pfizer as TICOVAC) for use in persons aged ≥1 year. A TBE Vaccine Work Group was formed in September 2020 to discuss use of TBE vaccine in U.S. adults and children. The policy question developed by the Work Group was “Should TBE vaccine be recommended for use in persons aged ≥1 year traveling to or residing in TBE risk areas and in laboratory staff working with TBE virus?” The critical outcomes were considered to be protection from disease after the 3-dose primary series and after the booster dose administered at 3 years after the primary series, and serious adverse events following vaccination. A systematic review of evidence was conducted as described in the methods section below. For protection after the primary series, findings were based on 10 observational studies and for protection after the booster dose, on two observational studies. Seropositivity within approximately 1 month of the primary series in adults and children was high with seropositivity rates ≥96% in all but one study. Similarly, seropositivity was high (100%) at one month after a booster dose and remained high (≥85%) in adults and children through 10 years after the booster dose. The evidence for both outcome measures was certainty level 3 (low) with indirectness a limitation but the magnitude of effect being a strength of the evidence. Findings for vaccine-related serious adverse events were based on results from four randomized controlled trials, nine observational studies, and three post-marketing surveillance studies. Vaccine-related serious adverse events were rarely reported. For this outcome measure, the certainty was level 2 (moderate); the evidence available from randomized controlled trials was downgraded because of risk of bias from inadequate blinding. The Work Group presented the GRADE assessment to the Advisory Committee on Immunization Practices (ACIP) as part of the Evidence to Recommendations (EtR) Framework at the January 12, 2022 ACIP meeting.
Introduction
On August 13, 2021, the FDA approved a TBE vaccine (manufactured by Pfizer as TICOVAC) for use in persons aged ≥1 years. This inactivated TBE vaccine is the first TBE vaccine to be licensed in the United States; other TBE vaccines are available internationally, but are not licensed in the United States. As Pfizer’s TBE vaccine is the only currently-available TBE vaccine in the United States, it will be referred to in this document as “the TBE vaccine” (or when necessary, “the FDA-approved TBE vaccine”).
The TBE vaccine has an adult formulation for individuals aged ≥16 years and a pediatric formulation for children and adolescents aged 1–15 years. The vaccination schedule includes 3 primary doses and 1 booster dose at ≥3 years after the primary series. The only difference between the adult and pediatric schedules is the interval between doses 1 and 2 which for adults is 14 days–3 months and for children and adolescents is 1–3 months. Dose 3 of the primary schedule is delivered at 5–12 months after dose 2 for both adults and children.
TBE is focally endemic in a geographic region extending from western and northern Europe through to eastern and northern Asia. TBE virus is primarily transmitted to humans by infected ticks. Clinical manifestations of TBE can include febrile illness or neurologic disease, including meningitis, encephalitis, or meningoencephalomyelitis. Since 2001, 20 TBE cases have been diagnosed among U.S. residents who traveled abroad to parts of Europe, Russia and China.1
An earlier formulation of Pfizer’s TBE vaccine was first licensed in Austria in 1976. Changes to the formulation over time have included removal of thimerosal and a transition of the production virus seed from mouse brain suspension to chick embryo fibroblast cells. The current adult (0.5mL) and pediatric (0.25mL) formulations became available in 2001 and 2003, respectively. Because of the manufacturing changes that have occurred, only the current formulation of the vaccine was considered in this GRADE assessment. TBE vaccine currently is marketed in about 30 countries, primarily in Europe. Since 2001, >75 million doses have been administered in Europe, about two-thirds of those to adults and one-third to children.
There are no efficacy data for the TBE vaccine and low TBE incidence would make such trials infeasible. There also are no field effectiveness studies for the FDA-approved TBE vaccine alone. One study from Austria showed a vaccine effectiveness estimate of 99% during a period when 90%–95% of the vaccine in use was the FDA-approved TBE vaccine, but the data were not considered in the GRADE assessment as other limitations included that subjects were vaccinated according to the Austrian schedule and many persons would have received the older TBE vaccine formulation.2 In the absence of relevant data on vaccine protection against TBE disease, this GRADE assessment considered immunogenicity data. Anti-TBE virus neutralizing antibodies are believed to confer protection against disease in humans, and a 50% neutralizing antibody titer of ≥10 is generally used in studies for other flavivirus vaccines to indicate protection.3 However, no formal correlate of protection has been established and no international reference reagents are available.
An ACIP TBE Vaccine Work Group was formed in September 2020 to discuss use of TBE vaccine among U.S. adults and children. The Work Group developed the following policy question “Should TBE vaccine be recommended for use in persons aged ≥1 year traveling to or residing in TBE risk areas and in laboratory staff working with TBE virus?” The components of the question (i.e., population, intervention, comparison, and outcomes [PICO]) are presented in Table 1. The outcomes that were considered for the TBE vaccine GRADE assessment and their rankings are presented in Table 2. A systematic review of evidence on vaccine safety and immunogenicity was conducted and the results are presented below. The GRADE assessment was summarized for ACIP at the January 12, 2022 ACIP meeting as part of the EtR Framework for TBE vaccine.
Methods
A systematic review was conducted for evidence for TBE vaccine for the pre-specified outcomes. To identify published literature that contained relevant evidence, we conducted a search of Medline, Embase, CINAHL, Scopus, and Cochrane Library databases for papers published from January 1995 through September 21, 2021. We used the following search strategy and terms: “Encephalitis, Tick-borne and (vaccin* or immunization* or Immuno AG or Baxter* or Pfizer*)” OR “(Tick-borne encephalitis OR TBE) and (vaccin* or immunization* or Immuno AG or Baxter* or Pfizer*)” OR “FSME-Immun*” OR “TICOVAC” (Appendix 1). Duplicate publications were identified and removed. We reviewed the title, abstract, or complete paper to identify relevant articles.
Published articles were included if they met the following criteria: 1) Involved human subjects; 2) Reported primary data; 3) Included data relevant to the outcome measures being assessed (i.e., vaccine efficacy, effectiveness, immunogenicity, or serious adverse events); 4) Included data for an FDA-approved dose and schedule; 5) Included data where all doses administered were with the current formulation of the vaccine; and 6) Reported results based on neutralizing antibody testing (for immunogenicity outcomes). Publications or data within publications were excluded if they represented a single case report, related to investigation of subjects with a medical condition which could affect safety or immunogenicity results (e.g., immunosuppression, post-thymectomy), or were only available in a non-English language.
We identified 1,737 unique publications using the search strategy. Among these publications, 1,717 were excluded as they did not meet one or more of the inclusion criteria, leaving 20 publications with relevant data4567891011121314151617181920212223 (Appendix 1). In addition to published studies we also searched the ClinicalTrials.gov website, reviewed FDA documents related to licensure of TBE vaccine (https://www.fda.gov/vaccines-blood-biologics/ticovac), and requested unpublished or other relevant data from the manufacturer. Evidence type was assessed through a review of the studies and their design, risk of bias, inconsistency, indirectness, imprecision, and other considerations (i.e., publication bias, strength of association, dose response, or opposing plausible residual confounding).
Results
Among the 20 publications, nine were included for evidence of protection and serious adverse events56791011121416, three were included for protection alone81822, and eight were included for serious adverse events alone413151719202123 (Appendix 1).
Protection after the 3-dose primary series
The evidence used to evaluate protection from disease after the 3-dose primary series was from 11 publications describing 10 studies567910111214161822 (Tables 3a and 3b). All were observational studies and all measured seropositivity after a primary series and not prevention of disease. Among 1,951 adult and pediatric subjects in 10 studies from whom seropositivity data were available after a primary series, 1,911 (98%) were seropositive at approximately 1 month (range: 1–6 weeks) after the primary series (Table 3a). The only study with a seropositivity rate <96% was a small study in which 35 subjects had testing conducted with a non-standard diagnostic test (i.e., neutralizing antibodies were measured with a rapid fluorescent focus inhibition test that used a non-vaccine-type TBE virus). Among 595 adult and pediatric subjects in two of the studies from whom seropositivity data were available at 3 years after the primary series1116, immediately prior to a booster dose, 574 (96%) were seropositive; seropositivity was 94% for adults and 98% for children (Table 3b).
Protection after the booster dose
The evidence used to evaluate protection from disease after the booster dose was from two studies described in three publications81116 (Tables 3c, 3d, and 3e). Both studies were observational and measured seropositivity and not prevention of disease. At 1 month, 5 years, and 10 years after a booster dose, the percentage of adults seropositive was 100% (240 of 240), 94% (209 of 222), and 85% (189 of 222), respectively. For children at the same time points, seropositivity rates were 100% (172 of 172), 99% (155 of 156), and 90% (140 of 155).
Serious adverse events
The evidence used to evaluate serious adverse events was from 16 studies described in 17 publications, including four randomized, controlled trials, nine observational studies, and three post-marketing surveillance studies45679101112131415161719202123 (Tables 3f, 3g, and 3h). Studies were conducted in Europe and Russia over a 20-year period, so one general limitation was that a standardized case definition was not used and it was not possible to seek clarification when a case definition was not provided. Among the 3,562 adult and 3,350 pediatric subjects who received ≥1 dose of vaccine in the primary series in four randomized controlled9152123 and nine observational studies456710121417, there were no serious adverse events considered vaccine-related reported (Table 3f). When data from the four randomized controlled trials were combined and weighted using a random effects model, there was no significant difference in proportions of subjects with serious adverse events within 1 month of a primary series dose of TBE vaccine or the comparator TBE vaccines (Figure 1). Among 240 adult and 202 pediatric subjects who received a booster dose of vaccine in two of the observational studies1116, no serious adverse events considered vaccine-related were reported (Table 3g). Finally, among 687 adult and 1,992 pediatric subjects who received a primary series dose or booster dose in three active post-marketing surveillance studies with follow-up limited to 4–6 days, there was one serious adverse event considered vaccine-related by study investigators131920 (Table 3h). A child aged 12 months with concomitant rhinopharyngitis, gastroenteritis, and otitis media was hospitalized for fever at 1 day after vaccination and had a febrile convulsion the following day.
Conclusion
Following the GRADE approach, we determined the quality of evidence for the pre-specified outcomes (i.e., protection after a 3-dose primary series and after a booster dose, and serious adverse events). Based on results from 10 observational studies with data at 1 month after the 3-dose primary series, seropositivity was high (≥96%) in all but one study. When subjects in one of adult and one of the pediatric studies were followed up at 3 years after the primary series (i.e., through to the time point when a booster dose is recommended), seropositivity remained high (≥94%). Two observational studies with data after a booster dose administered at 3 years, including one adult and one pediatric cohort, demonstrated that seropositivity rates were high at 1 month (100%), 5 years (≥94%), and 10 years (≥85%) later. For evidence on both outcomes measures of protection after vaccination, the certainty was level 3 (low) based on 1) results being from observational studies only; 2) indirectness because the likelihood of protection from disease was based on seropositivity with no established correlate of protection and there being likely but unconfirmed protection against non-European TBE virus subtypes; and 3) upgrading because of the magnitude of effect (Tables 4 and 5).
Based on results from four randomized controlled trials, nine observational studies, and three post-marketing surveillance studies, vaccine-related serious adverse events were rarely reported. For this outcome measure, the certainty was level 2 (moderate). Data were available from randomized controlled trials which provide a higher quality of evidence but evidence type was downgraded because of the risk of bias from inadequate blinding (Tables 4 and 5).
Table 1: Policy Question and PICO
Policy question
Should TBE vaccine be recommended for use in persons aged ≥1 year traveling to or residing in TBE risk areas and in laboratory staff working with TBE virus
Population
Persons aged ≥1 year traveling to or residing in TBE risk areas and laboratory staff working with TBE virus.
Intervention
Vaccination with TBE vaccine according to recommended doses and schedules*
Comparison
No TBE vaccination*
Outcomes
- Protection from disease after the 3-dose primary series
- Protection from disease after a booster dose at 3 years following the primary series
- Serious adverse events
*With advice on tick bite prevention measures for persons traveling abroad
Table 2: Outcomes considered for TBE vaccine GRADE and rankings
| Outcome | Importancea | Included in evidence profile |
|---|---|---|
| Prevention of disease after a 3-dose primary series | Critical | Yes* |
| Prevention of disease after a booster dose† | Critical | Yes* |
| Serious adverse events | Critical | Yes |
| Systemic reactions | Important but not critical | No |
| Local reactions | Not important for decision-making | No |
| Interference when co-administered with other vaccines | Important but not critical | No |
*Indirect evidence for prevention of disease from immunogenicity data as no efficacy or effectiveness data
†Administered at 3 years after a primary series
Table 3a: Summary of studies reporting seropositivity by neutralizing antibody testing at approximately 1 month (range: 1–6 weeks)1 after a 3-dose primary series of TBE vaccine
References in this table:56791011141822
| Author, year | Age group | Study type | No. (%) subjects seropositive2 after FDA-approved TBE vaccine | No. (%) subjects seropositive2 after comparator TBE vaccine | Study limitations | ||
|---|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | ||||
| Ehrlich 2003 [5] | Adults (16–65 yrs) | Obs | 114/118 | (97)3,4 | -- | -- | |
| Loew-Baselli 2006/FDA5 [9] | Adults (16–64 yrs) | Obs | 411/416 | (99)3 | -- | -- | |
| Loew-Baselli 2006(b)/FDA6 [10] | Adults (16–65 yrs) | Obs | 44/44 | (100) | -- | -- | Dose 3 administered at a slightly longer than recommended interval (13–14 months) |
| Loew-Baselli 2011/FDA7 [11] | Adults (16–79 yrs) | Obs | 295/297 | (99)8 | -- | -- | Dose 2 for some subjects was administered at a slightly shorter than recommended interval (10–13 days) |
| Wanke 2012 [22] | Adults (≥70 yrs)9 | Obs | 136/137 | (99) | -- | -- | |
| Hertzell 201610 [7] | Adults (18–59 yrs) | Obs | 27/35 | (77) | -- | -- | Non-standard diagnostic test (rapid fluorescent focus inhibition test with non-vaccine-type TBE virus) used to measure neutralizing antibodies with 50% effective dose ≥5 considered seropositive |
| Harrison 2020 [6] | Adults (21–60 yrs) | Obs | 10/10 | (100) | -- | -- | |
| Pöllabauer 2010/FDA11 [14] | Children (1–15 yrs) | Obs | 388/406 | (96)3 | -- | -- | Dose 2 for some subjects administered at a slightly shorter than recommended interval based on pediatric schedule (21-29 days) |
| Pöllabauer 2010/FDA11 [14] | Children (1–15 yrs) | Obs | 358/360 | (99)12 | -- | -- | Dose 2 for some subjects was administered at a slightly shorter than recommended interval based on pediatric schedule (21-29 days) |
| Prymula 2012/FDA14 [18] | Children (1–11 yrs) | Obs13 | 128/128 | (100) | -- | -- | |
FDA=Food & Drug Administration; Obs=Observational study
1Seropositivity was assessed at 1 month after dose 3 unless specifically indicated
2Anti-TBE virus neutralizing antibody titer ≥10
3Assessed at 21–28 days after dose 3
4Testing with a 100% neutralization test and titers >10 considered positive
5Publication by Loew-Baselli 2006 supplemented by data from Package Insert (TICOVAC)
6Publication by Loew-Baselli 2006(b) supplemented by data from FDA Clinical Review Memo
7Data were included in a publication on the clinical development of TBE vaccine but were not available in a separate publication, and results are based on FDA’s Clinical Review Memo of study 690601 (Clinicaltrials.gov: NCT00460486)
8Assessed at 21 days after dose 3
9Age range of participants not available
10Results presented are for the subjects in healthy control group and <60 years
11Publication by Pöllabauer 2010 supplemented by data from Package Insert (TICOVAC) and/or FDA Clinical Review Memo
12Assessed at 35–42 days after dose 3
13Randomized, controlled trial with no valid comparative immunogenicity data as dose 3 for the control group was the FDA-approved TBE vaccine
14Denominator is based on data from FDA Clinical Review Memo and is for subjects with baseline neutralizing antibody titer ≤10.
Table 3b. Summary of studies reporting seropositivity by neutralizing antibody testing at 3 years after a 3-dose primary series of TBE vaccine
| Author, year | Age group | Study type | No. (%) subjects seropositive1 after FDA-approved TBE vaccine | No. (%) subjects seropositive1 after comparator TBE vaccine | Study limitations | ||
|---|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | ||||
| Loew-Baselli 2009/FDA2 [11] | Adults (16–65 yrs) | Obs | 229/243 | (94) | -- | -- | |
| Poellabauer 2019 [16] | Children (1–15 yrs) | Obs | 345/352 | (98) | -- | -- | Dose 2 for some subjects was administered at a slightly shorter than recommended interval based on pediatric schedule (21-29 days) |
FDA=Food & Drug Administration; Obs=Observational study
1Anti-TBE virus neutralizing antibody titer ≥10
2Publication by Loew-Baselli 2009 supplemented by data from FDA Clinical Review Memo and manufacturer for subjects who received the FDA-approved TBE vaccine alone
Table 3c. Summary of studies reporting seropositivity by neutralizing antibody testing at 1 month after a booster dose of TBE vaccine administered at 3 years after the primary series
| Author, year | Age group | Study type | No. (%) subjects seropositive1 after FDA-approved TBE vaccine | No. (%) subjects seropositive1 after comparator TBE vaccine | Study limitations | ||
|---|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | ||||
| Loew-Baselli 2009/FDA2 [11] | Adults (18–67 yrs) | Obs | 240/240 | (100)3 | -- | -- | |
| Poellabauer 2019 [16] | Children (3–18 yrs) | Obs | 172/172 | (100)3,4 | -- | -- | Subjects were aged 1–15 years at the time of enrollment in the initial primary series study so some (approximately 21%) were aged >15 years at the time of the booster dose and received an adult dose for the booster vaccination |
FDA=Food & Drug Administration; Obs=Observational study
1Anti-TBE virus neutralizing antibody titer ≥10
2Publication by Loew-Baselli 2009 supplemented by data from FDA Clinical Review Memo and manufacturer for subjects who received the FDA-approved TBE vaccine alone
3Serum collected at 21–35 days after booster vaccination
4An additional 30 subjects had a booster dose administered at 4 years (n=29) or at 5 years (n=1) and 100% were seropositive at 1 month after the booster
Table 3d. Summary of studies reporting seropositivity by neutralizing antibody testing at 5 years after a booster dose of TBE vaccine
| Author, year | Age group | Study type | No. (%) subjects seropositive1 after FDA-approved TBE vaccine | No. (%) subjects seropositive1 after comparator TBE vaccine | Study limitations | ||
|---|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | ||||
| Konior 2017/Pfizer2 [8] | Adults (18–67 yrs) | Obs | 209/222 | (94) | -- | -- | |
| Poellabauer 20193 [16] | Children (3–18 yrs) | Obs | 155/156 | (99) | -- | -- | Subjects were aged 1–15 years at the time of enrollment in the initial primary series study so some (approximately 21%) were aged >15 years at the time of the booster dose and received an adult dose for the booster vaccination |
FDA=Food & Drug Administration; Obs=Observational study
1Anti-TBE virus neutralizing antibody titer ≥10
2Publication by Konior 2017 supplemented by data from Pfizer on subjects who received the FDA-approved TBE vaccine alone
3Booster dose was administered at intervals after the primary series of 3, 4, or 5 years for approximately 85%, 14%, and <1% of children, respectively
Table 3e. Summary of studies reporting seropositivity by neutralizing antibody testing at 10 years after a booster dose of TBE vaccine
| Author, year | Age group | Study type | No. (%) subjects seropositive1 after FDA-approved TBE vaccine | No. (%) subjects seropositive1 after comparator TBE vaccine | Study limitations | ||
|---|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | ||||
| Konior 2017/Pfizer2 [8] | Adults (18–67 yrs) | Obs | 189/222 | (85) | -- | -- | |
| Poellabauer 20193 [16] | Children (3–18 yrs) | Obs | 140/155 | (90) | -- | -- | Subjects were aged 1–15 years at the time of enrollment in the initial primary series study so some (approximately 21%) were aged >15 years at the time of the booster dose and received an adult dose for the booster vaccination |
FDA=Food & Drug Administration; Obs=Observational study
1Anti-TBE virus neutralizing antibody titer ≥10
2Publication by Konior 2017 supplemented by data from Pfizer on subjects who received the FDA-approved TBE vaccine alone
3Booster dose was administered at intervals after the primary series of 3, 4, or 5 years for approximately 85%, 14%, and <1% of children, respectively
Table 3f. Summary of studies reporting serious adverse events (SAE)1 reported within one month after any dose of the primary series of TBE vaccine
References in this table:4567910121415172123
| Author, year | Age group | Study type | No. (%) subjects with SAE after FDA-approved TBE vaccine | No. (%) subjects with SAE after comparator TBE vaccine2 | Study limitations | ||
|---|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | ||||
| Loew-Baselli 2006/FDA3 [9] | Adults (16–64 yrs) | RCT | 0/2977 | (0) | 0/975 | (0) | Data after doses 1 and 2 only |
| Wittermann 2009 [23] | Children (1–10 yrs) | RCT | 0/168 | (0) | 0/166 | (0) | Data after doses 1 and 2 only; Dose 2 for some subjects was administered at a slightly shorter than recommended interval based on pediatric schedule (14 days) |
| Pöllabauer 2010(b) [15] | Children (1–11 yrs) | RCT | 0/150 | (0) | 0/152 | (0) | Data after doses 1 and 2 only |
| Vorovitch 2019 [21] | Children (1–15 yrs) | RCT | 0/42 | (0) | 0/49 | (0) | Data after doses 1 and 2 only |
| Ehrlich 2003 [5] | Adults (16–65 yrs) | Obs | 0/135 | (0)4 | -- | -- | |
| Loew-Baselli 2006(b)/FDA5 [10] | Adults (16–65 yrs) | Obs | 0/60 | (0) | -- | -- | |
| Loew-Baselli 2011/FDA6 [12] | Adults (16–79 yrs) | Obs | 0/340 | (0) | -- | -- | Dose 2 for some subjects was administered at a slightly shorter than recommended interval (10–13 days) |
| Hertzell 2016 [7] | Adults (18–59 yrs) | Obs | 0/35 | (0) | -- | -- | |
| Harrison 2020 [6] | Adults (21–60 yrs) | Obs | 0/15 | (0) | -- | -- | |
| Barrett 2003/FDA7 [4] | Children (1–12 yrs) | Obs | 0/101 | (0) | -- | -- | Dose 2 for some subjects administered at a slightly shorter than recommended interval based on pediatric schedule (14–29 days) |
| Prelog 2008 [17] | Children (1–15 yrs) | Obs | 0/52 | (0) | -- | -- | |
| Pöllabauer 2010 [14] | Children (1–15 yrs) | Obs | 0/420 | (0)8 | -- | -- | Dose 2 for some subjects administered at a slightly shorter than recommended interval based on pediatric schedule (21-29 days) |
| Pöllabauer 2010 [14] | Children (1–15 yrs) | Obs | 0/2417 | (0) | -- | -- | Dose 2 for some subjects was administered at a slightly shorter than recommended interval based on pediatric schedule (21-29 days) |
FDA=Food & Drug Administration; RCT=Randomized controlled trial; Obs=Observational study
1SAEs considered vaccine-related by study investigators
2Inactivated TBE vaccine (Encepur with polygeline, which has since been replaced by a polygeline-free formulation) based on European TBE virus strain and licensed by Chiron Behring GmbH, Germany (now Bavarian Nordic)[Loew-Baselli 2006], Inactivated TBE vaccine (Encepur Children) based on European TBE virus strain and licensed by Novartis Vaccines (now Bavarian Nordic)[Wittermann 2009, Pöllabauer 2010(b)], Inactivated TBE vaccine (Tick-E-Vac) based on Far Eastern TBE virus strain and manufactured by Chumakov Federal Scientific Center (Russia)[Vorovitch 2019]
3Publication by Loew-Baselli 2006 supplemented by data from FDA Clinical Review Memo. Two SAEs were considered unrelated by study investigators, who likely had access to additional information, but could not be excluded as possibly related to vaccination based on publicly reported data. An 18-year-old female was diagnosed with viral meningitis commencing 9 days after dose 1, and a 32-year-old female died an unavailable number of days after receipt of dose 1 from cardiac arrest and at autopsy was shown to have a previously unrecognized congenital heart defect with interstitial pneumonia and myocarditis of probable viral etiology.
4Results from adults who received 2.4µg (n=135) dose, but also 0 vaccine-related SAEs among adults who received 0.6µg (n=137) or 1.2µg (n=133) dose
5Publication by Loew-Baselli 2006(b) supplemented by data from FDA Clinical Review Memo
6Data only in review article and results are supplemented by data from FDA’s Clinical Review Memo (study 690601; Clinicaltrials.gov: NCT00460486)
7Data only in review article and results are supplemented by data from FDA Clinical Review Memo (studies 198 and 215)
8Results represent children who received the 1.2µg (n=420) dose, but there also were 0 vaccine-related SAEs among children who received a 0.6µg (n=436) or 0.3µg (n=422) dose.
Table 3g. Summary of studies reporting serious adverse events (SAE)1 reported within 1 month after a booster dose of TBE vaccine
| Author, year | Age group | Study type | No. (%) subjects with SAE after FDA-approved TBE vaccine | No. (%) subjects with SAE after comparator TBE vaccine | Study limitations | ||
|---|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | ||||
| Loew-Baselli 2009/FDA2 [11] | Adults (18–67 yrs) | Obs | 0/240 | (0) | -- | -- | Final study visit was at 21–35 days after booster vaccination |
| Poellabauer 20193 [16] | Children (3–18 yrs) | Obs | 0/202 | (0) | -- | -- | |
FDA=Food & Drug Administration; Obs=Observational study
1SAEs considered vaccine-related by study investigators
2Publication by Loew-Baselli 2009 supplemented by data from FDA Clinical Review Memo for subjects who received the FDA-approved TBE vaccine alone
3Booster dose was administered at intervals after the primary series of 3 years for 85% (n=172), 4 years for 14% (n=29) and 5 years for <1% (n=1)
Table 3h. Summary of post-marketing surveillance (PMS) studies reporting serious adverse events (SAE)
References in this table:131920
| Author, year | Age group | Study type | No. (%) patients with SAE after FDA-approved TBE vaccine | No. (%) patients with SAE after comparator TBE vaccine | Study limitations | ||
|---|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | ||||
| Pavlova 20031 [13] | Children (1–12 yrs) | Obs/PMS | 1/14322,3 | (<1) | NR | NR | Subjects under surveillance for only 4 days |
| Satz 20054 [19] | Children (1–15 yrs) | Obs/PMS | 0/560 | (0) | NR | NR | Subjects under surveillance for only 6 days; Publication did not document lack of SAEs but because other local and systemic adverse events were comprehensively documented, lack of SAEs was assumed |
| Satz 2005(b)5 [20] | Adults (≥18 yrs) | Obs/PMS | 0/687 | (0) | NR | NR | Subjects under surveillance for only 6 days |
FDA=Food & Drug Administration; Obs=Observational surveillance study; PMS=Post-marketing surveillance; NR=Not relevant
1Active surveillance conducted at 110 medical centers. The TBE vaccine used for children was half an adult dose (half of the liquid vaccine in the pre-filled syringe was discarded prior to administration) and was identical to the pediatric vaccine subsequently available in 2002. Only dose 1 was administered during the surveillance period and surveillance was conducted on days 0–3 post-vaccination
2Overall, 1,899 doses were administered but 467 doses were administered to children aged 6 months through <1 year which is outside the FDA-approved age range for the vaccine; however, among these 467 infants, 0 SAEs were reported
3The one report considered possibly vaccine-related by the study investigator was for a subject aged 12 months with concomitant rhinopharyngitis, gastroenteritis, and otitis media who was hospitalized for fever (1 day after vaccination) and had a febrile convulsion the following day (2 days after vaccination)
4Active surveillance with 560 doses administered to 490 subjects. Data were gathered through telephone interview at 5 days post-vaccination. Publication did not document lack of SAEs but because other local and systemic adverse events were comprehensively documented, lack of SAEs was assumed
5Active surveillance conducted at 15 centers. In total, 687 doses were administered to 570 subjects and data were gathered through telephone interview at 5 days post-vaccination
Figure 1. Pooled risk ratio for serious adverse events (SAE)1 reported within 1 month after any dose of the primary series of TBE vaccine in randomized controlled trials2
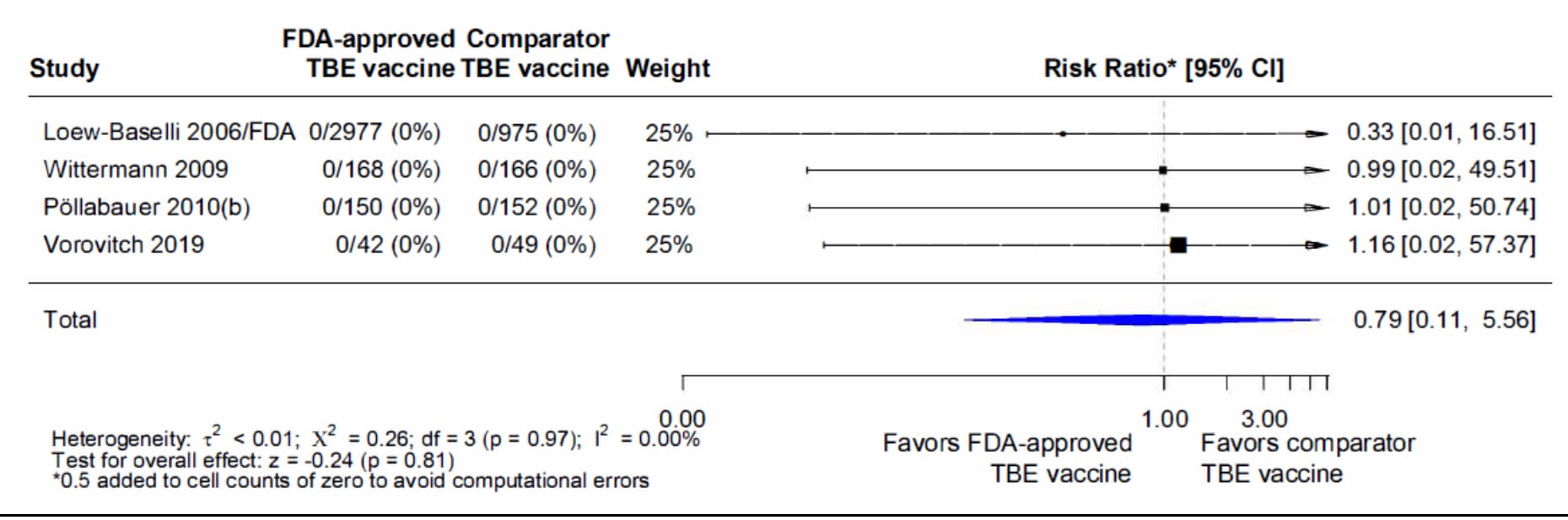
1SAEs considered vaccine-related by study investigators
2Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion with SAE in FDA-approved TBE vaccine group / Proportion with SAE in comparator TBE vaccine group. Risk ratio <1.0 favors FDA-approved TBE vaccine versus other TBE vaccine
Table 4: Grade Summary of Findings Table
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design1 | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations2 | Intervention | Comparison | Relative risk (95% CI) |
Absolute risk (95% CI) |
||
| Prevention of disease after the 3-dose primary series | ||||||||||||
| 0 | Randomized studies | - | - | - | - | - | - | - | - | - | - | - |
| 103 | Nonrandomized studies | Not serious | Not serious | Serious4 | Not serious | Yes5 | 19516 | 0 | - | - | 3 | Critical |
| Prevention of disease after a booster dose administered at 3 years after the primary series | ||||||||||||
| 0 | Randomized studies | - | - | - | - | - | - | - | - | - | - | - |
| 27 | Nonrandomized studies | Not serious | Not serious | Serious4 | Not serious | Yes5 | 4126 | 0 | - | - | 3 | Critical |
| Serious adverse events | ||||||||||||
| 4 | Randomized studies | Serious8 | None | None | Not serious | - | 3337 | 1342 | 0.8 (0.1–5.6) |
NC | 2 | Critical |
| 129 | Nonrandomized studies | Not serious | Not serious | None | Not serious | - | 625410 | 0 | - | - | 3 | Critical |
NC=Not calculated (NB. Comparator vaccines included 3 different TBE vaccines or vaccine formulations so extensive statistical comparison of the FDA-approved TBE vaccine with this group of vaccines is not appropriate)
1Randomized controlled trials start at evidence level 1 and observational studies at level 3
2Other considerations include publication bias, strength of association, dose response, or opposing plausible residual confounding
310 studies described in 11 publications
4Seropositivity and not protection from disease was measured, there is no formal serologic correlate of protection, and there is likely but unconfirmed protection against non-European TBE virus subtypes (i.e., Far Eastern, Siberian)
5Magnitude of effect supports upgrading the quality of evidence
6Based on number of subjects with immunogenicity data at 1 month after primary series or 1 month after booster dose
72 studies described in 3 publications
8Risk of bias due to lack of blinding of study personnel in three of the four studies
912 studies (including observational and post-marketing surveillance studies) described in 13 publications
10Based on number of subjects with safety data within 1 month after a primary series dose or in a post-marketing surveillance study
Table 5. Summary of Evidence for Outcomes of Interest
| Outcome | Importance | Included in profile | Certainty |
|---|---|---|---|
| Prevention of disease after the 3-dose primary series | Critical | Yes | 3 |
| Prevention of disease after a booster dose | Critical | Yes | 3 |
| Serious adverse events | Critical | Yes | 2 |
Appendix 1. Studies Included in the Review of Evidence
References in this table:4567891011121314151617181920212223
| Publication | Study design | Country | Age group | N intervention | N comparison | Outcomes |
|---|---|---|---|---|---|---|
| Barrett 2003 [4] | Obs | Austria | Children (1–12 yrs) | 101 | - | Safety (primary series) |
| Erlich 2003 [5] | Obs | Belgium | Adults (16–65 yrs) | 118 | - | Immunogenicity after primary series (1 month) |
| 135 | Safety (primary series) | |||||
| Harrison 2020 [6] | Obs | Austria | Adults (21–60 yrs) | 10 | - | Immunogenicity after primary series (1 month) |
| 15 | Safety (primary series) | |||||
| Hertzell 2016 [7] | Obs | Sweden | Adults (18–59 yrs) | 35 | - | Immunogenicity after primary series (1 month) |
| 35 | Safety (primary series) | |||||
| Konior 2017 [8] | Obs | Poland | Adults (18–67 yrs) | (222)1 | Immunogenicity after booster dose (5 years, 10 years) | |
| Loew-Baselli 2006 [9] | Obs | Poland | Adults (16–64 yrs) | 416 | - | Immunogenicity after primary series (1 month) |
| RCT | 2977 | 975 | Safety (primary series) | |||
| Loew-Baselli 2006(b) [10] | Obs | Belgium | Adults (16–65 yrs) | 44 | - | Immunogenicity after primary series (1 month) |
| 60 | Safety (primary series) | |||||
| Loew-Baselli 2009 [11] | Obs | Poland | Adults (16–65 yrs) | (243)1 | - | Immunogenicity after primary series (3 yrs) |
| Adults (18–67 yrs) | (240)1 | Immunogenicity after booster dose (1 month) | ||||
| Adults (18–67 yrs) | (240)1 | Safety (booster dose) | ||||
| Loew-Baselli 2011 [12] | Obs | Poland | Adults (16–79 yrs) | 297 | - | Immunogenicity after primary series (1 month) |
| 340 | Safety (primary series) | |||||
| Pavlova 2003 [13] | Obs/PMS | Austria | Children (1–12 yrs) | 1432 | - | Safety (post-marketing surveillance) |
| Pöllabauer 2010 (2 studies) [14] | Obs (study 1) |
Austria, Germany | Children (1–15 yrs) | 406 | - | Immunogenicity after primary series (1 month) |
| 420 | Safety (primary series) | |||||
| Obs (study 2) |
Poland, Austria, Germany | 360 | - | Immunogenicity after primary series (1 month) | ||
| 2417 | Safety (primary series) | |||||
| Pöllabauer 2010(b) [15] | RCT | Czech Republic, Austria | Children (1–11 yrs) | 150 | 152 | Safety (primary series) |
| Poellabauer 2019 [16] | Obs | Poland, Austria, Germany | Children (1–15 yrs) | (352)2 | - | Immunogenicity after primary series (3 yrs) |
| Children (3–18 yrs) | (155–172)2 | Immunogenicity after booster dose (1 month, 5 years, 10 years) | ||||
| Children (3–18 yrs) | (202)2 | Safety (booster dose) | ||||
| Prelog 2008 [17] | Obs | Austria | Children (1–15 yrs) | 52 | - | Safety (primary series) |
| Prymula 2012 [18] | Obs (RCT with no valid comparative immunogenicity data as dose 3 for the control group was the FDA-approved TBE vaccine) | Czech Republic, Austria | Children (1–11 yrs) | 128 | - | Immunogenicity after primary series (1 month) |
| Satz 2005 [19] | Obs/PMS | Switzerland | Children (1–15 yrs) | 560 | - | Safety (post-marketing surveillance) |
| Satz 2005(b) [20] | Obs/PMS | Switzerland | Adults (≥18 yrs) | 687 | - | Safety (post-marketing surveillance) |
| Vorovitch 2019 [21] | RCT | Russia | Children (1–15 yrs) | 42 | 49 | Safety (primary series) |
| Wanke 2012 [22] | Obs | Switzerland | Adults (≥70 yrs) | 137 | - | Immunogenicity after primary series (1 month) |
| Witterman 2009 [23] | RCT | Germany | Children (1–10 yrs) | 168 | 166 | Safety (primary series) |
Obs=Observational study; RCT=Randomized, controlled trial; PMS=Post-marketing surveillance
1Subset of subjects from Loew-Baselli 2006
2Subset of subjects from Pöllabauer 2010 (study 2)
View the complete list of GRADE evidence tables
- Hills SL, Broussard KR, Broyhill JC, Shastry LG, Cossaboom CM, White JL, Machesky KD, Kosoy O, Girone K, Klena JD, Backenson BP, Gould CV. Lind L, Hieronimus A, Gaines DN, Wong SJ, Choi MJ, Laven JL, Staples JE, Fischer M. Tick-borne encephalitis among US travelers, 2010–2020. J Travel Med 2022;29:1–6.
- Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 2007;25:7559–67.
- Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2-3 September, 2004. Vaccine 2005;23:5205–11.
- Barrett PN, Schober-Bendixen S, Ehrlich HJ. History of TBE vaccines. Vaccine 2003;21 Suppl 1:S41–9.
- Ehrlich HJ, Pavlova BG, Fritsch S, Poellabauer EM, Loew-Baselli A, Obermann-Slupetzky O, Maritsch F, Cil I, Dorner F, Barrett PN. Randomized, phase II dose-finding studies of a modified tick-borne encephalitis vaccine: evaluation of safety and immunogenicity. Vaccine 2003;22:217–23.
- Harrison N, Grabmeier-Pfistershammer K, Graf A, Schwarzinger I, Aberle JH, Stiasny K, Greinix H, Rabitsch W, Kalhs P, Ramharter M, Burgmann H, Forstner C. Humoral immune response to tick-borne encephalitis vaccination in allogeneic blood and marrow graft recipients. NPJ Vaccines 2020; 5(1):67.
- Hertzell KB, Pauksens K, Rombo L, Knight A, Vene S, Askling HH. Tick-borne encephalitis (TBE) vaccine to medically immunosuppressed patients with rheumatoid arthritis: A prospective, open-label, multi-centre study. Vaccine 2016;34:650–5.
- Konior R, Brzostek J, Poellabauer EM, Jiang Q, Harper L, Erber W. Seropersistence of TBE virus antibodies 10 years after first booster vaccination and response to a second booster vaccination with FSME-IMMUN 0.5mL in adults. Vaccine 2017;35:3607–13.
- Loew-Baselli A, Konior R, Pavlova BG, Fritsch S, Poellabauer E, Maritsch F, Harmacek P, Krammer M, Barrett PN, Ehrlich HJ, FSME-IMMUN study group. Safety and immunogenicity of the modified adult tick-borne encephalitis vaccine FSME-IMMUN: results of two large phase 3 clinical studies. Vaccine 2006;24:5256–63.
- Loew-Baselli A, Poellabauer EM Fritsch S, Koska M, Vartian N, Himly C, Harmacek P, Maritsch F, Pavlova B, Ehrlich H. Immunogenicity and safety of FSME-IMMUN 0.5 ml using a rapid immunization schedule. Int J Med Micro 2006(b);296(S1):213–14.
- Loew-Baselli A, Poellabauer EM, Pavlova BG, Fritsch S, Koska M, Bobrovsky R, Konior R, Ehrlich HJ. Seropersistence of tick-borne encephalitis antibodies, safety and booster response to FSME-IMMUN 0.5 ml in adults aged 18-67 years. Hum Vaccin 2009;5:551–6.
- Loew-Baselli A, Poellabauer EM, Pavlova BG, Fritsch S, Firth C, Petermann R, Barrett PN, Ehrlich HJ. Prevention of tick-borne encephalitis by FSME-IMMUN vaccines: review of a clinical development programme. Vaccine 2011;29:7307–19.
- Pavlova BG, Loew-Baselli A, Fritsch S, Poellabauer EM, Vartian N, Rinke I, Ehrlich HJ. Tolerability of modified tick-borne encephalitis vaccine FSME-IMMUN “NEW” in children: results of post-marketing surveillance. Vaccine 2003;21:742–5 (Pfizer study 197).
- Poellabauer E, Angermayr R, Behre U, Zhang P, Harper L, Schmitt HJ, Erber W. Seropersistence and booster response following vaccination with FSME-IMMUN in children, adolescents, and young adults. Vaccine 2019;37:3241–50.
- Pöllabauer EM, Fritsch S, Pavlova BG, Löw-Baselli A, Firth C, Koska M, Maritsch F, Barrett PN, Ehrlich HJ. Clinical evaluation to determine the appropriate paediatric formulation of a tick-borne encephalitis vaccine. Vaccine 2010;28:4558–65.
- Pöllabauer EM, Pavlova BG, Löw-Baselli A, Fritsch S, Prymula R, Angermayr R, Draxler W, Firth C, Bosman J, Valenta B, Harmacek P, Maritsch F, Barrett PN, Ehrlich HJ. Comparison of immunogenicity and safety between two paediatric TBE vaccines. Vaccine 2010(b);28:4680–5.
- Prelog M, Wilk C, Keller M, Karall T, Orth D, Geiger R, Walder G, Laufer G, Cottogni M, Zimmerhackl Lothar B, Stein J, Grubeck-Loebenstein B, Wuerzner R. Diminished response to tick-borne encephalitis vaccination in thymectomized children. Vaccine 2008;26:595–600.
- Prymula R, Pöllabauer EM, Pavlova BG, Löw-Baselli A, Fritsch S, Angermayr R, Geisberger A, Barrett PN, Ehrlich HJ. Antibody persistence after two vaccinations with either FSME-IMMUN® Junior or ENCEPUR® Children followed by third vaccination with FSME-IMMUN® Junior. Hum Vaccin Immunother 2012;8:736–42. (Clinicaltrials.gov: NCT00840801).
- Satz N. The safety of a TBE-vaccine in children: a post-marketing surveillance study in Switzerland. Hospitalis 2005;75(7/8):319–22.
- Satz N. A post-marketing surveillance study on the safety of a vaccine for tickborne encephalitis. Hospitalis 2005(b);75(9):319–22.
- Vorovitch MF, Maikova GB, Cherokhaeva LL, Romanenko VV, Karganova GG, Ishmukhametov AA. Comparison of the immunogenicity and safety of two pediatric TBE vaccines based on the Far Eastern and European virus subtypes. Adv Virol 2019;2019: :5323428.
- Wanke K, Von Braun A, Haberli L, Mekker A, Steffen P, Stiasny K, Heinz F, Unger B, Karrer U. Immunogenicity and safety of tick-borne encephalitis vaccination in healthy elderly individuals. Clin Microbiol Infect 2012;18:246 (poster presentation). (Clinicaltrials.gov: NCT00461695).
- Wittermann C, Schöndorf I, Gniel D. Antibody response following administration of two paediatric tick-borne encephalitis vaccines using two different vaccination schedules. Vaccine 2009;27:1661–6 (Note: also published as Wittermann C, Nicolay U, Hilbert AK, Schoendorf I. Paediatric tick-borne encephalitis (TBE) vaccines: Schedules to optimize protection. Int J Med Microbiol 2008;298(S1):301–04.
- Food and Drug Administration (FDA). Clinical Review Memo – TICOVAC and Package Insert — TICOVAC. August 13, 2021. Available at https://www.fda.gov/vaccines-blood-biologics/ticovac.
