About
CDC vaccine recommendations are developed using an explicit evidence-based method based on the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Introduction
On February 14, 2025, the U.S. Food and Drug Administration approved the use of a pentavalent meningococcal vaccine (MenACWY-CRM/MenB-4C [Penmenvy, GSK]) for prevention of invasive disease caused by Neisseria meningitidis serogroups A, B, C, W, and Y among persons aged 10–25 years. On April 16, 2025, CDC's Advisory Committee on Immunization Practices (ACIP) recommended MenACWY-CRM/MenB-4C may be used when both MenACWY and MenB are indicated at the same visit for 1) healthy persons aged 16–23 years (routine schedule) when shared clinical decision-making favors administration of MenB vaccine and 2) persons aged ≥10 years who are at increased risk for meningococcal disease (e.g., because of persistent complement deficiencies, complement inhibitor use or functional or anatomic asplenia).
A systematic review and Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used to inform ACIP’s deliberations regarding use of MenACWY-CRM/MenB-4C. The policy questions under consideration (Tables 1a–c) were:
- Should the pentavalent vaccine be included as an option for MenACWY/MenB vaccination in people currently recommended to receive both vaccines?
- Should the pentavalent vaccine be included as an option for MenACWY vaccination in people currently recommended to receive only MenACWY vaccine?
- Should the pentavalent vaccine be included as an option for MenB vaccination in people currently recommended to receive only MenB vaccine?
Methods
Members of ACIP's Meningococcal Vaccines Work Group ("Work Group") selected policy questions, specified relevant outcomes, and rated the importance of specified outcomes (including benefits and harms) before the GRADE assessment. Tables 1a–c present the population, intervention, comparison, and outcome(s) (PICO) relevant for each policy question, while Table 2 presents the judged importance of specified outcomes. All Work Group members and CDC staff participating in the GRADE assessment met ACIP requirements for reporting conflicts of interest.
A systematic literature search was conducted using Medline, Embase, Global Health, CINAHL, Cochrane, Scopus, and clinicaltrials.gov databases from 2015 to July 2024. Duplicates were identified using the Endnote “Find Duplicates” function set to match on title, author, and year.1 Efforts were also made to obtain unpublished or other relevant data. Two reviewers independently screened titles and abstracts and reviewed full-text records in Microsoft Excel.2 Records were included if they presented primary immunogenicity or safety data on GSK’s pentavalent meningococcal vaccine. Characteristics of all records meeting inclusion criteria are shown in Appendix 1.
Records were then excluded if 1) GSK’s pentavalent meningococcal vaccine was only administered as a booster dose following two doses of experimental pentavalent vaccine or currently licensed MenB vaccine, or 2) if no relevant comparator groups were included (either a single dose of currently licensed MenACWY vaccine or ≥1 dose of currently licensed MenB vaccine). For studies included in the final assessment, specific trial arm regimens, number of participants in each trial arm, and presence of information about specified outcomes are shown in Appendix 2.
The strategy for evidence search and retrieval is in Appendix 3. When multiple studies contributed data to the GRADE assessment for a specified outcome, a random-effects meta-analysis was used to generate estimates of relative and absolute risk across all contributing studies.
Results
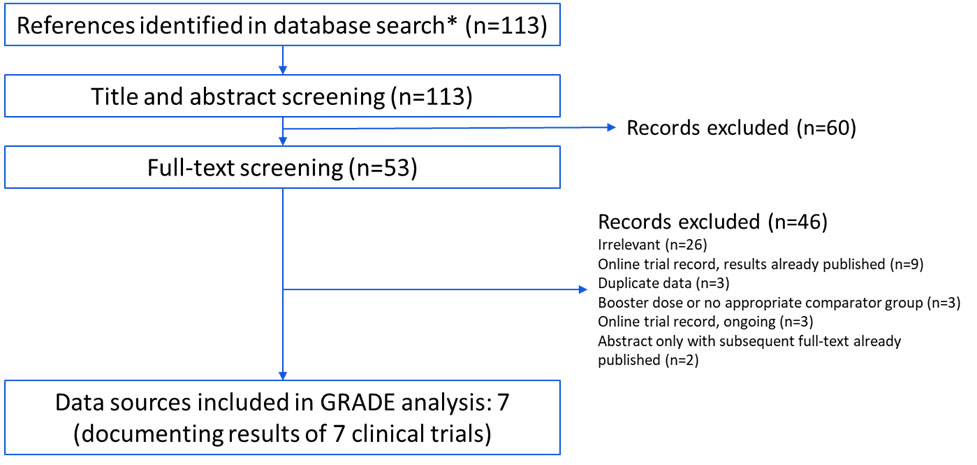
A summary of the GRADE assessment was presented to ACIP on October 24, 2024 and reviewed on April 16, 2025. Overall, 113 records were identified and screened. Sixty records were excluded based on the title and abstract, and 46 records were excluded based on full-text review (Appendix 3). Three of the records excluded during full-text review met inclusion criteria (i.e., they presented primary data on GSK's pentavalent meningococcal vaccine) but either only administered GSK's pentavalent meningococcal vaccine as a booster dose or had no relevant comparator group for the policy questions under consideration.345 The remaining seven records described results from seven randomized controlled trials,6789101112 of which all but one were blinded.10 These records were included in the evidence synthesis and GRADE assessment (Appendix 2). Data on the specified outcomes of interest (Table 2) are summarized in Tables 3a–3d, and the GRADE assessment is summarized in Tables 4a and 4b. When the available data applied to multiple policy questions, only one harmonized table is shown; separate sub-tables were created when the data only applied to 1 or 2 policy questions. For example, study data on short-term immunogenicity regarding serogroups A, B, C, W, and Y are presented in Table 3a, which is split into two sub-tables: one sub-table for serogroup A, C, W, and Y immunogenicity (applicable to PICOs 1 and 2) and another for serogroup B immunogenicity (applicable to PICOs 1 and 3). Two outcomes — meningococcal disease caused by serogroups A, B, C, W, and Y and interferences with other recommended vaccines administered concurrently — lacked data (Table 2).
Summary
For all outcomes with identified data, the initial evidence level was high because the available data were from randomized controlled clinical trials. Summaries of the final evidence certainty for each PICO are presented in Tables 5a–5c. The evidence certainty for short-term immunity against serogroups A, B, C, W, and Y (PICOs 1–3) was moderate for healthy persons and low for persons at increased risk. The evidence certainty for persistent immunity against serogroup B (PICOs 1 and 3) was low for healthy persons and very low for persons at increased risk. No evidence was available for persistent immunity against serogroups A, C, W, and Y (PICOs 1 and 2).
For serious adverse events possibly related to study vaccination, the evidence certainty was moderate for healthy persons and low for persons at increased risk. The evidence certainty for non-serious adverse events varied by PICO. When comparing MenABCWY to concomitant administration of MenACWY and MenB (PICO 1) or to administration of only MenACWY (PICO 2), the evidence certainty was moderate for healthy persons and low for persons at increased risk. When comparing MenABCWY to administration of only MenB (PICO 3), the evidence certainty was high for healthy persons and moderate for persons at increased risk.
Table 1a: Policy Question and PICO 1
Policy Question
Should the pentavalent vaccine be included as an option for MenACWY/MenB vaccination in people currently recommended to receive both vaccines?
Population
All individuals aged ≥10 years currently recommended to receive MenACWY and MenB vaccines
Intervention
Vaccination with the pentavalent vaccine
Comparison
Vaccination with currently licensed MenACWY and MenB vaccines
Outcomes
- Disease caused by serogroups A, B, C, W, and Y
- Short-term immunity
- Persistent immunity
- Interference with other recommended vaccines administered concurrently
- Serious adverse events
- Non-serious adverse events
Table 1b: Policy Question and PICO 2
Policy Question
Should the pentavalent vaccine be included as an option for MenACWY vaccination in people currently recommended to receive only MenACWY vaccine?
Population
All individuals aged ≥10 years currently recommended to receive MenACWY vaccine
Intervention
Vaccination with the pentavalent vaccine
Comparison
Vaccination with currently licensed MenACWY vaccine
Outcomes
- Disease caused by serogroups A, B, C, W, and Y
- Short-term immunity
- Persistent immunity
- Interference with other recommended vaccines administered concurrently
- Serious adverse events
- Non-serious adverse event
Table 1c: Policy Question and PICO 3
Policy Question
Should the pentavalent vaccine be included as an option for MenB vaccination in people currently recommended to receive only MenB vaccine?
Population
All individuals aged ≥10 years currently recommended to receive MenB vaccine
Intervention
Vaccination with the pentavalent vaccine
Comparison
Vaccination with currently licensed MenB vaccine
Outcomes
- Disease caused by serogroups B
- Short-term immunity
- Persistent immunity
- Interference with other recommended vaccines administered concurrently
- Serious adverse events
- Non-serious adverse events
Table 2: Outcomes and Rankings
| Outcome | Importance | Included in Evidence Profile |
|---|---|---|
| Meningococcal disease caused by serogroups A, B, C, W, and Y | Critical | Yesa |
| Short-term immunity | Critical | Yes |
| Persistent immunity | Important | Yes |
| Interference with other recommended vaccines administered concurrently | Important | Yesa |
| Serious adverse events | Critical | Yes |
| Non-serious adverse events | Important | Yes |
Table 2 Footnote
aThis outcome was included in the final list of outcomes to evaluate in the evidence assessment; however, data for this outcome was lacking.
Table 3a: Summary of Studies Reporting Short-Term Immunity — PICOs 1, 2, and 3
| Intervention | Comparator | Author, year or Study ID | Population | Serogroup | Time since last study dose | Percent (N) Intervention | Percent (N) Comparator | Study limitations (risk of bias) |
|---|---|---|---|---|---|---|---|---|
| Seroprotection based on hSBA titersa for serogroups A, C, W, and Y | ||||||||
| MenABCWY (1 dose) | MenACWY-CRM (1 dose) | Saez-Llorens 2015b,c | 11–18 years; ACWY naïve |
A | Baseline | 4% (82) | 5% (82) | Not serious |
| 1 month | 80% (79) | 88% (80) | ||||||
| C | Baseline | 38% (82) | 32% (81) | |||||
| 1 month | 93% (80) | 84% (81) | ||||||
| W | Baseline | 68% (82) | 68% (82) | |||||
| 1 month | 100% (79) | 98% (82) | ||||||
| Y | Baseline | 28% (82) | 40% (82) | |||||
| 1 month | 91% (79) | 100% (83) | ||||||
| Beran 2021d,e | 10–25 years; ACWY naïve |
A | Baseline | 2.1% (98) | 3.1% (97) | Not serious | ||
| 1 month | 65.3% (98) | 68.8% (97) | ||||||
| C | Baseline | 45.9% (98) | 41.7% (97) | |||||
| 1 month | 89.6% (98) | 86.6% (97) | ||||||
| W | Baseline | 21.9% (98) | 26.3% (97) | |||||
| 1 month | 71.9% (98) | 66.0% (97) | ||||||
| Y | Baseline | 6.1% (98) | 12.5% (97) | |||||
| 1 month | 83.2% (98) | 85.4% (97) | ||||||
| MenACWY-CRM + MenB-4C administered in same arm (1 dose) | A | Baseline | 1.0% (101) | |||||
| 1 month | 73.5% (101) | |||||||
| C | Baseline | 48.0% (101) | ||||||
| 1 month | 94.1% (101) | |||||||
| W | Baseline | 26.3% (101) | ||||||
| 1 month | 70.6% (101) | |||||||
| Y | Baseline | 11.0% (101) | ||||||
| 1 month | 81.8% (101) | |||||||
| MenACWY-CRM + MenB-4C administered in different arms (1 dose) | A | Baseline | 3.2% (97) | |||||
| 1 month | 80.2% (97) | |||||||
| C | Baseline | 51.5% (97) | ||||||
| 1 month | 92.8% (97) | |||||||
| W | Baseline | 28.7% (97) | ||||||
| 1 month | 74.2% (97) | |||||||
| Y | Baseline | 11.6% (97) | ||||||
| 1 month | 88.4% (97) | |||||||
| MenACWY-CRM (1 dose) | V72_72f,g | 10–25 years; ACWY naïve or primed |
A | Baseline | 9.2% (1,452) | 11.7% (137) | Not serious | |
| 1 month | 79.5% (132) | 90.2% (133) | ||||||
| C | Baseline | 29.8% (1,487) | 28.8% (139) | |||||
| 1 month | 74.8% (139) | 64.0% (136) | ||||||
| W | Baseline | 12.6% (1,473) | 12.9% (140) | |||||
| 1 month | 80.3% (142) | 69.3% (137) | ||||||
| Y | Baseline | 12.2% (1,489) | 13.5% (141) | |||||
| 1 month | 82.2% (146) | 80.0% (140) | ||||||
| MenABCWY_019f,h | 15–25 years; ACWY primed |
A | Baseline | 27.7% (546) | 28.8% (549) | Not serious | ||
| 1 month | 98.3% (605) | 98.1% (585) | ||||||
| C | Baseline | 57.7% (601) | 56.2% (584) | |||||
| 1 month | 98.9% (609) | 99.0% (593) | ||||||
| W | Baseline | 36.3% (597) | 33.4% (583) | |||||
| 1 month | 98.4% (607) | 96.8% (592) | ||||||
| Y | Baseline | 37.5% (600) | 34.9% (576) | |||||
| 1 month | 97.9% (606) | 97.6% (591) | ||||||
| Seroprotection based on hSBA titers for serogroup B | ||||||||
| MenABCWY (2 doses at 0,6 months) | MenB-4C (2 doses at 0,6 months)i | V72_72g,i | 10–25 years; B naïve |
fHbp | Baseline | 5.4% (762) | 3.4% (730) | Not serious |
| 1 month | 95.9% (738) | 94.6% (707) | ||||||
| NadA | Baseline | 6.2% (780) | 4.4% (731) | |||||
| 1 month | 96.2% (734) | 98.0% (707) | ||||||
| NHBA | Baseline | 18.5% (764) | 20.9% (731) | |||||
| 1 month | 95.3% (738) | 97.5% (711) | ||||||
| PorA | Baseline | 2.1% (751) | 1.4% (716) | |||||
| 1 month | 75.3% (709) | 82.6% (684) | ||||||
Table 3A Footnotes
Table 3b: Summary of Studies Reporting Persistent Immunity — PICOs 1, 2, and 3
| Intervention | Comparator | Author, year or Study ID | Population | Serogroup | Time since last study dose | Percent (N) Intervention | Percent (N) Comparator | Study limitations (risk of bias) |
|---|---|---|---|---|---|---|---|---|
| Seroprotection based on hSBA titersa for serogroups A, C, W, and Y | ||||||||
| No relevant studies | ||||||||
| Seroprotection based on hSBA titersa for serogroup B | ||||||||
| MenABCWY (2 doses at 0,6 months) | MenB-4C (2 doses at 0,2 months) | Vesikari 2021b | 10–18 years; B naïve |
fHbp | Two years | 26% (70) | 18% (119) | Not serious |
| NadA | 74% (72) | 82% (121) | ||||||
| NHBA | 37% (71) | 28% (122) | ||||||
| PorA | 18% (71) | 16% (121) | ||||||
Table 3B Footnotes
Table 3c: Summary of Studies Reporting Serious Adverse Events Assessed as Possibly Related to Vaccinationa — PICOs 1, 2, and 3
| Author, year or Study ID | Intervention | Comparator | Population | Event window | n/Nb Intervention | n/Nb Comparator | Study limitations (risk of bias) |
|---|---|---|---|---|---|---|---|
| Saez-Llorens 2015 | MenABCWY (2 doses at 0,2 months) |
MenACWY-CRM (1 dose) | 11–18 years | 91 days | 0/83 | 0/83 | Not serious |
| Block 2015 | 10–25 years | 241 days | 0/120 | 0/121 | Not serious | ||
| MenB-4C (2 doses at 0,2 months) |
0/122 | ||||||
| Welsch 2018 | MenACWY-CRM (1 dose) | 10–18 years | 6 months | 0/150 | 0/146 | Not serious | |
| Vesikari 2021 | MenB-4C (2 doses at 0,2 months) |
10–18 years | 13 months | 1/228c | 0/221 | Not serious | |
| MenABCWY (2 doses at 0,6 months) |
0/129 | ||||||
| MenABCWY (3 doses at 0,2,6 months) |
0/157 | ||||||
| MenABCWY (2 doses at 0,1 months) |
1/151d | ||||||
| MenABCWY (2 doses at 0,11 months) |
0/147 | ||||||
| MenABCWY primary series (2 doses at 0,2 months) with 2-year booster dose |
MenB-4C primary series (2 doses at 0,2 months) with 2-year booster dose |
12–20 years | 3 months | 0/127 | 0/126 | ||
| MenABCWY primary series (2 doses at 0,6 months) with 2-year booster dose |
0/74 | ||||||
| MenABCWY primary series (3 doses at 0,2,6 months) with 2-year booster dose |
0/77 | ||||||
| MenABCWY (2 doses at 0,2 months) |
MenB-4C (2 doses at 0,2 months) |
12–20 years | 3 months | 0/99 | 0/101 | ||
| Beran 2021 | MenABCWY (2 doses at 0,2 months) |
MenACWY-CRM + MenB-4C administered in same arm (2 doses at 0,2 months) | 10–25 years | 91 days | 0/100 | 0/104 | Not serious |
| MenACWY-CRM + MenB-4C administered in different arms (2 doses at 0,2 months) |
0/100 | ||||||
| MenB-4C (2 doses at 0,2 months) |
1/94e | ||||||
| MenACWY-CRM (1 dose) | 0/102 | ||||||
| v72_72f | MenABCWY (2 doses at 0,6 months)g |
MenB-4C (3 doses at 0,2,6 months) |
10–25 years | 361 days | 1/1,657h | 0/897 | Not serious |
| MenB-4C (2 doses at 0,6 months) |
1/906i | ||||||
| MenACWY-CRM (1 dose) | 1/178j | ||||||
| MenABCWY_019f | 10–25 years | 361 days | 0/626 | 0/621 | Not serious |
Table 3C Footnotes
bIf a specific denominator was not provided, these denominators reflect the number of participants enrolled. Studies had little, if any, participant attrition.
Table 3d: Summary of Studies Reporting Nonserious Adverse Eventsa — PICOs 1, 2, and 3
| Author, year or Study ID | Intervention | Comparator | Population | n/Nb Intervention | n/Nb Comparator | Study limitations (risk of bias) |
|---|---|---|---|---|---|---|
| Solicited non-serious adverse events after receiving the first planned study vaccine dose | ||||||
| Saez-Llorens 2015 | MenABCWY (2 doses at 0,2 months) |
MenACWY-CRM (1 dose) | 11–18 years | 79/83 | 56/82 | Not serious |
| Block 2015 | 10–25 years | ≥92/107 | ≥39/92c | Not serious | ||
| MenB-4C (2 doses at 0,2 months) |
≥103/114 | |||||
| Welsch 2018 | MenACWY-CRM (1 dose) | 10–18 years | ≥121/147 | ≥49/135c | Not serious | |
| Vesikari 2021 | MenB-4C (2 doses at 0,2 months) |
10–18 years | ≥223/231 | ≥213/227 | Not serious | |
| MenABCWY (2 doses at 0,6 months) |
≥126/134 | |||||
| MenABCWY (3 doses at 0,2,6 months) |
≥146/159 | |||||
| MenABCWY (2 doses at 0,1 months) |
≥142/154 | |||||
| MenABCWY (2 doses at 0,11 months) |
≥135/144 | |||||
| MenABCWY (2 doses at 0,2 months)d |
MenB-4C (2 doses at 0,2 months)d |
12–20 years | ≥94/99 | ≥93/101 | ||
| Beran 2021 | MenABCWY (2 doses at 0,2 months) |
MenACWY-CRM + MenB-4C administered in same arm (2 doses at 0,2 months) | 10–25 years | 93/100 | 102/104 | Not serious |
| MenACWY-CRM + MenB-4C administered in different arms (2 doses at 0,2 months) |
97/100 | |||||
| MenB-4C (2 doses at 0,2 months) |
93/94 | |||||
| MenACWY-CRM (1 dose) | 76/102 | |||||
| v72_72e | MenABCWY (2 doses at 0,6 months) |
MenB-4C (3 doses at 0,2,6 months) |
10–25 years | ≥1,503/1,638 | ≥807/885 | Not serious |
| MenB-4C (2 doses at 0,6 months) |
≥819/894 | |||||
| MenACWY-CRM (1 dose) | ≥78/178 | |||||
| MenABCWY_019f | 10–25 years | ≥486/608 | ≥222/600 | Not serious | ||
| Solicited non-serious adverse events after completing a real-world vaccine seriesg | ||||||
| Vesikari 2021 | MenABCWY (2 doses at 0,6 months) |
MenB-4C (2 doses at 0,2 months)h |
10–18 years | ≥106/120 | ≥193/212 | Not serious |
| MenABCWY (2 doses at 0,11 months) |
≥119/132 | |||||
| v72_72e | MenABCWY (2 doses at 0,6 months) |
MenB-4C (2 doses at 0,6 months) |
10–25 years | ≥1,258/1,428 | ≥676/759 | Not serious |
| MenB-4C (2 of 3 doses at 0,2,6 months) |
≥714/823 | |||||
| MenB-4C (3 of 3 doses at 0,2,6 months) |
≥677/765 | |||||
| MenACWY-CRM (1 dose)i | ≥78/178 | |||||
| MenABCWY_019f | 10–25 years | ≥377/507 | ≥222/600 | Not serious | ||
Table 3D Footnotes
aNonserious adverse events are adverse events that did not meet the definition of a serious adverse event and may range in severity. Solicited adverse events were used as a surrogate for nonserious adverse events.
Table 4a: GRADE Summary of Findings for Healthy Persons — PICOs 1, 2, and 3
| Certainty Assessment | No. of patients | Effecta | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistencyb | Indirectness | Imprecision | Other considerations | GSK MenABCWY | Comparator | Relative (95% CI) |
Absolute (95% CI) per 100,000 |
||
| Short-term immunity after one dose vs MenACWY-CRM (follow-up: 1 month) | ||||||||||||
| 4c | Randomized trials | Not serious | Not serious | Seriousd | Not serious | GSK funded | Serogroup A | Moderate | Critical | |||
| 914 | 1,093 | 0.94 (0.86, 1.01) |
5,437 fewer (11,705 fewer to 832 more) | |||||||||
| Serogroup C | ||||||||||||
| 926 | 1,105 | 1.03 (0.97, 1.10) |
2,726 more (2,545 fewer to 7,997 more) | |||||||||
| Serogroup W | ||||||||||||
| 926 | 1,106 | 1.02 (1.00, 1.04) |
1,930 more (314 to 3,546 more) | |||||||||
| Serogroup Y | ||||||||||||
| 929 | 1,109 | 0.98 (0.93, 1.03) |
1,930 fewer (6,528 fewer to 2,668 more) | |||||||||
| Short-term immunity after two doses vs MenB-4C (follow-up: 1 month) | ||||||||||||
| 1 | Randomized trials | Not serious | None | Seriousd | Not serious | GSK funded | fHbp | Moderate | Critical | |||
| 738 | 707 | 1.01 (0.99, 1.04) |
1,300 more (896 fewer to 3,496 more) | |||||||||
| NadA | ||||||||||||
| 734 | 707 | 0.98 (0.96, 1.00) |
1,800 fewer (3,526 to 74 fewer) | |||||||||
| NHBA | ||||||||||||
| 738 | 711 | 0.98 (0.96,1.00) | 2,200 fewer (4,110 to 290 fewer) | |||||||||
| PorA | ||||||||||||
| 709 | 684 | 0.91 (0.86, 0.96) |
7,300 fewer (11,560 to 3,040 fewer) | |||||||||
| Persistent immunity after one dose vs MenACWY-CRM | ||||||||||||
| 0 | Important | |||||||||||
| Persistent immunity after two doses vs MenB-4C (follow-up: 2 years) | ||||||||||||
| 1 | Randomized trials | Not serious | None | Seriousd | Seriouse | GSK funded | fHbp | Low | Important | |||
| 70 | 119 | 1.46 (0.84, 2.54) |
8,000 more (4,379 fewer to 20,379 more) | |||||||||
| NadA | ||||||||||||
| 72 | 121 | 0.90 (0.77, 1.06) |
8,000 fewer (20,228 fewer to 4,228 more) | |||||||||
| NHBA | ||||||||||||
| 71 | 122 | 1.31 (0.86, 2.00) |
9,000 more (4,770 fewer to 22,770 more) | |||||||||
| PorA | ||||||||||||
| 71 | 121 | 1.17 (0.61, 2.22) |
2,000 more (9,069 fewer to 13,069 more) | |||||||||
| Serious adverse events assessed as related to vaccination after any dose | ||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Not serious | Seriousf | GSK funded | 3,925 (0-2 events per study) | 3,922 (0-2 events per study) | 1.08 (0.31, 3.77) |
6 fewer (150 fewer to 138 more) | Moderate | Critical |
| Non-serious adverse events after one dose | ||||||||||||
| 4 | Randomized trials | Not serious | vs MenB-4C (one dose) | Important | ||||||||
| Not serious | Not serious | Not serious | GSK funded | 2,766 | 2,315 | 0.99 (0.96, 1.02) |
1,148 fewer (4,123 fewer to 1,827 more) | High | ||||
| 1 | vs MenB-4C + MenACWY-CRM (one dose each) | |||||||||||
| None | Not serious | Seriouse | GSK funded | 100 | 204 | 0.95 (0.90, 1.01) |
4,549 fewer (9,981 fewer to 883 more) | Moderate | ||||
| 6 | vs MenACWY-CRM (one dose) | |||||||||||
| Not serious | Not serious | Seriousf | GSK funded | 2,683 | 1,189 | 1.80 (1.46, 2.22) |
37,892 more (28,361 to 47,423 more) | Moderate | ||||
| Non-serious adverse events after ≥2 doses | ||||||||||||
| 2 | Randomized trials | Not serious | vs MenB-4C (≥2 doses) | Important | ||||||||
| Not serious | Not serious | Not serious | GSK funded | 1,680 | 2,559 | 1.00 (0.98, 1.02) |
213 fewer (2,196 fewer to 1,770 more) | High | ||||
| 2 | vs MenACWY-CRM (one dose) | |||||||||||
| Not serious | Not serious | Seriousf | GSK funded | 1,935 | 778 | 2.01 (1.83, 2.21) |
40,317 more (33,610 to 47,023 more) | Moderate | ||||
Table 4A Footnotes
Table 4b: GRADE Summary of Findings for Persons at Increased Risk due to Underlying Medical Conditions — PICOs 1, 2, and 3
| Certainty Assessment | No. of patients | Effecta | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistencyb | Indirectness | Imprecision | Other considerations | GSK MenABCWY | Comparator | Relative (95% CI) |
Absolute (95% CI) per 100,000 |
||
| Short-term immunity after one dose vs MenACWY-CRM (follow-up: 1 month) | ||||||||||||
| 4c | Randomized trials | Not serious | Not serious | Very seriousd,e | Not serious | GSK funded | Serogroup A | Low | Critical | |||
| 914 | 1,093 | 0.94 (0.86, 1.01) |
5,437 fewer (11,705 fewer to 832 more) | |||||||||
| Serogroup C | ||||||||||||
| 926 | 1,105 | 1.03 (0.97, 1.10) |
2,726 more (2,545 fewer to 7,997 more) | |||||||||
| Serogroup W | ||||||||||||
| 926 | 1,106 | 1.02 (1.00, 1.04) |
1,930 more (314 to 3,546 more) | |||||||||
| Serogroup Y | ||||||||||||
| 929 | 1,109 | 0.98 (0.93, 1.03) |
1,930 fewer (6,528 fewer to 2,668 more) | |||||||||
| Short-term immunity after two doses vs MenB-4C (follow-up: 1 month) | ||||||||||||
| 1 | Randomized trials | Not serious | None | Very seriousd,e | Not serious | GSK funded | fHbp | Low | Critical | |||
| 738 | 707 | 1.01 (0.99, 1.04) | 1,300 more (896 fewer to 3,496 more) | |||||||||
| NadA | ||||||||||||
| 734 | 707 | 0.98 (0.96, 1.00) | 1,800 fewer (3,526 to 74 fewer) | |||||||||
| NHBA | ||||||||||||
| 738 | 711 | 0.98 (0.96,1.00) | 2,200 fewer (4,110 to 290 fewer) | |||||||||
| PorA | ||||||||||||
| 709 | 684 | 0.91 (0.86, 0.96) | 7,300 fewer (11,560 to 3,040 fewer) | |||||||||
| Persistent immunity after one dose vs MenACWY-CRM | ||||||||||||
| 0 | Important | |||||||||||
| Persistent immunity after two doses vs MenB-4C (follow-up: 2 years) | ||||||||||||
| 1 | Randomized trials | Not serious | None | Very seriousd,e | Seriousf | GSK funded | fHbp | Very low | Important | |||
| 70 | 119 | 1.46 (0.84, 2.54) | 8,000 more (4,379 fewer to 20,379 more) | |||||||||
| NadA | ||||||||||||
| 72 | 121 | 0.90 (0.77, 1.06) | 8,000 fewer (20,228 fewer to 4,228 more) | |||||||||
| NHBA | ||||||||||||
| 71 | 122 | 1.31 (0.86, 2.00) | 9,000 more (4,770 fewer to 22,770 more) | |||||||||
| PorA | ||||||||||||
| 71 | 121 | 1.17 (0.61, 2.22) | 2,000 more (9,069 fewer to 13,069 more) | |||||||||
| Serious adverse events assessed as related to vaccination after any dose | ||||||||||||
| 7 | Randomized trials | Not serious | Not serious | Seriouse | Seriousg | GSK funded | 3,925 (0-2 events per study) | 3,922 (0-2 events per study) | 1.08 (0.31, 3.77) |
6 fewer (150 fewer to 138 more) | Low | Critical |
| Non-serious adverse events after one dose | ||||||||||||
| 4 | Randomized trials | Not serious | vs MenB-4C (one dose) | Important | ||||||||
| Not serious | Seriouse | Not serious | GSK funded | 2,766 | 2,315 | 0.99 (0.96, 1.02) |
1,148 fewer (4,123 fewer to 1,827 more | Moderate | ||||
| 1 | vs MenB-4C + MenACWY-CRM (one dose each) | |||||||||||
| None | Seriouse | Seriousf | GSK funded | 100 | 204 | 0.95 (0.90, 1.01) | 4,549 fewer (9,981 fewer to 883 more) | Low | ||||
| 6 | vs MenACWY-CRM (one dose) | |||||||||||
| Not serious | Seriouse | Seriousg | GSK funded | 2,683 | 1,189 | 1.80 (1.46, 2.22) | 37,892 more (28,361 to 47,423 more) | Low | ||||
| Non-serious adverse events after ≥2 doses | ||||||||||||
| 2 | Randomized trials | Not serious | vs MenB-4C (≥2 doses) | Important | ||||||||
| Not serious | Seriouse | Not serious | GSK funded | 1,680 | 2,559 | 1.00 (0.98, 1.02) | 213 fewer (2,196 fewer to 1,770 more) | Moderate | ||||
| 2 | vs MenACWY-CRM (one dose) | |||||||||||
| Not serious | Seriouse | Seriousg | GSK funded | 1,935 | 778 | 2.01 (1.83, 2.21) | 40,317 more (33,610 to 47,023 more) | Low | ||||
Table 4B Footnotes
aWhere >1 study is included in an outcome’s assessment, the presented effects and 95% confidence intervals (CIs) are derived from a random-effects meta-analysis. Where only 1 study is included, the presented 95% CIs are traditional Wald confidence intervals.
Table 5a: Summary of Evidence — PICO 1
| Outcome | Importance | Included in Evidence Profile | Certainty for Healthy Individuals | Certainty for Individuals at Increased Risk |
|---|---|---|---|---|
| Short-term immunity | Critical | Yes | Moderate | Low |
| Persistent immunity | Important | Yes | N/Aa, Low | N/A, Very Low |
| Serious adverse events | Critical | Yes | Moderate | Low |
| Non-serious adverse events | Important | Yes | Moderate, N/Ab | Low, N/Ab |
Table 5A Footnotes
Table 5b: Summary of Evidence — PICO 2
| Outcome | Importance | Included in Evidence Profile | Certainty for Healthy Individuals | Certainty for Individuals at Increased Risk |
|---|---|---|---|---|
| Short-term immunity | Critical | Yes | Moderate | Low |
| Persistent immunity | Important | No | N/Aa | N/Aa |
| Serious adverse events | Critical | Yes | Moderate | Low |
| Non-serious adverse events | Important | Yes | Moderate | Low |
Table 5B Footnotes
Table 5c: Summary of Evidence — PICO 3
| Outcome | Importance | Included in Evidence Profile | Certainty for Healthy Individuals | Certainty for Individuals at Increased Risk |
|---|---|---|---|---|
| Short-term immunity | Critical | Yes | Moderate | Low |
| Persistent immunity | Important | Yes | Low | Very Low |
| Serious adverse events | Critical | Yes | Moderate | Low |
| Non-serious adverse events | Important | Yes | High | Moderate |
Appendix 1: Potentially Relevant Studies Identified in Review of Evidence
| Study Registration(s) | Location(s) | Study Design | Phase | Blinding | Population | Author, year or Study ID | Period | Included in final GRADE assessment? |
|---|---|---|---|---|---|---|---|---|
| NCT01210885 NCT01367158 NCT02451514 |
Chile, Colombia, Panama | RCT | II | Observer-blind | Healthy, immuno-naïve individuals aged 11–18 years | Saez-Llorens 2015 | Dec 2010–Jul 2011 | Yes |
| Saez-Llorens 2015_2 | Jul 2011–Jul 2012 | No | ||||||
| Open-labela | Prior participants + individuals with no meningococcal vaccine history | Saez-Llorens 2018 | Jun–Dec 2015 | No | ||||
| NCT01272180 NCT01992536 |
Poland, USA | RCT | II | Observer-blind | Healthy, immuno-naïve individuals aged 10–25 years | Block 2015 | Aug 2011–Sep 2012 | Yes |
| Szenborn 2018 | Dec 2013–Apr 2015 | No | ||||||
| NCT02140762 NCT02285777 | USA | RCT | IIb | Observer-blind | Healthy, immuno-naïve individuals aged 10–18 years | Welsch 2018 | May 2014–Jun 2015 | Yes |
| NCT02212457 NCT02946385 |
Finland, Poland | RCT | IIb | Observer-blind | Healthy, immuno-naïve individuals aged 10–20 years | Vesikari 2021 | Aug 2014–Mar 2016 Nov 2016–Feb 2018 |
Yes |
| NCT03587207 | Czechia | RCT | II | Open-label | Healthy, immuno-naïve individuals aged 10–25 years | Beran 2021 | Jul–Dec 2018 | Yes |
| NCT04502693 | Australia, Canada, Czechia, Estonia, Finland, Turkey, USA | RCT | III | Observer-blind | Healthy individuals aged 10–25 years with no history of meningococcal disease or MenB vaccination | V72_72 | Aug 2020–Sep 2022 | Yes |
| NCT04707391 | Argentina, Australia, Canada, USA | RCT | III | Observer-blind | Healthy individuals aged 15–25 years with no meningococcal disease history vaccinated with MenACWY ≥4 years prior | MenABCWY_019 | Jan 2021–Sep 2023 | Yes |
Appendix 1 Footnotes
Appendix 2: Summary of Studies Included in Final GRADE Assessment
Three studies from Appendix 1 (Saez-Llorens 2015b, Saez-Llorens 2018, and Szenborn 2018) were excluded from all components of the final assessment. These studies either evaluated booster doses after a primary series of meningococcal vaccine (not relevant to the recommendations being considered) or evaluated primary series schedules not aligned with the recommendations being considered.
| Author, year | MenABCWY | Comparator(s) | MenABCWY vs Comparator Included in GRADE for... | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Regimen | # enrolled | # completed | Regimen | # enrolled | # completed | Short-Term Immunity | Long-Term Immunity | Adverse Events | |
| Saez-Llorens 2015a | 0,2 mos | 83 | 81 | 0 mos Q | 83 | 83 | Yes | No | Yes |
| Block 2015b | 0,2 mos | 120 | 103 | 2 mos Q | 121 | 107 | No | No | Yes |
| 0,2 mos B | 122 | 109 | No | No | Yes | ||||
| Welsch 2018 | 0,2 mos | 154 | 139 | 2 mos Q | 151 | 135 | No | No | Yes |
| Vesikari 2021 | 0,2 mos | 232 | 211 | 0,2 mos B | 228 | 228 | No | No | Yes |
| 0,6 mos | 134 | 123 | No | No | Yes | ||||
| 0,2,6 mos | 160 | 147 | No | No | Yes | ||||
| 0,1 mos | 157 | 141 | No | No | Yes | ||||
| 0,11 mos | 152 | 137 | No | No | Yes | ||||
| 0,2 mosc+P | 127 | 127 | 0,2 mosc+B | 126 | 126 | No | No | Yes | |
| 0,6 mosc+P | 74 | 74 | No | Yes | Yes | ||||
| 0,2 mosd | 101 | 99 | 0,2 mos Bd | 99 | 96 | No | No | Yes | |
| Beran 2021 | 0,2 mos | 100 | 100 | 0,2 mos QB/S | 104 | 104 | Yes | No | Yes |
| 0,2 mos QB/D | 100 | 100 | Yes | No | Yes | ||||
| 0,2 mos B | 94 | 94 | No | No | Yes | ||||
| 0 mos Q | 102 | 102 | Yes | No | Yes | ||||
| v72_72 | 0,6 mos | 1,657 | 1,497 | 0,2,6 mos B | 897 | 797 | No | No | Yes |
| 0 mos Q | 178 | 163 | Yes | No | Yes | ||||
| 0,6 mos B | 906 | 811 | Yes | No | Yes | ||||
| MenABCWY_019 | 0,6 mos | 626 | 541 | 0 mos Q | 621 | 542 | Yes | No | Yes |
Abbreviations: MenB vaccine dose (B); MenABCWY vaccine dose (P); MenACWY vaccine dose (Q)
Appendix 2 Footnotes
Appendix 3: Strategy for Evidence Search and Screening
Databases searched: Medline, Embase, Global Health, CINAHL, Cochrane, Scopus, and clinicaltrials.gov
Search terms
exp Neisseria meningitidis/ OR (Neisseria meningitidis OR meningococcal OR MenB-4C).ti,ab,kf.) AND pentavalent.ti,ab,kf.))
AND
vaccin*.ti,ab,kf. (in addition to clinical trials identifiers using "OR")
Efforts were made to obtain unpublished or other relevant data
Appendix 4: Summary of Studies Reporting Seroresponse
| Intervention | Comparator | Author, year or Study ID | Population | Serogroup | Percent (N) Intervention | Percent (N) Comparator | Study limitations (risk of bias) |
|---|---|---|---|---|---|---|---|
| Seroresponse based on hSBA titersa for serogroups A, C, W, and Y 1 month after 1 vaccine dose | |||||||
| MenABCWY | MenACWY-CRM | Saez-Llorens 2015b,c | 11–18 years; ACWY naïve |
A | 80% (79) | 86% (78) | Not serious |
| C | 74% (80) | 68% (78) | |||||
| W | 56% (79) | 60% (80) | |||||
| Y | 77% (79) | 78% (81) | |||||
| Beran 2021d | 10–25 years; ACWY naïve |
A | 61.7% (98) | 67.4% (97) | Not serious | ||
| C | 54.2% (98) | 52.1% (97) | |||||
| W | 60.6% (98) | 41.4% (97) | |||||
| Y | 77.9% (98) | 76.8% (97) | |||||
| MenACWY-CRM + MenB-4C administered in same arm | A | 71.3% (101) | |||||
| C | 46.5% (101) | ||||||
| W | 52.0% (101) | ||||||
| Y | 75.5% (101) | ||||||
| MenACWY-CRM + MenB-4C administered in different arms | A | 80.6% (97) | |||||
| C | 51.0% (97) | ||||||
| W | 51.6% (97) | ||||||
| Y | 78.3% (97) | ||||||
| MenACWY-CRM | V72_72e,f | 10–25 years; ACWY naïve or primed |
A | 74.0% (127) | 86.0% (129) | Not serious | |
| C | 66.9% (139) | 56.7% (134) | |||||
| W | 74.1% (139) | 66.2% (136) | |||||
| Y | 76.0% (146) | 72.1% (140) | |||||
| MenABCWY_019e,g | 15–25 years; ACWY primed |
A | 92.5% (509) | 95.0% (505) | Not serious | ||
| C | 94.0% (570) | 94.0% (546) | |||||
| W | 94.3% (565) | 93.9% (544) | |||||
| Y | 93.7% (567) | 94.4% (537) | |||||
| Seroresponse based on hSBA titersa for serogroup B 1 month after 2 vaccine doses | |||||||
| MenABCWY (2 doses at 0,6 months) | MenB-4C (2 doses at 0,6 months) | V72_72f,h | 10–25 years; B naïve |
fHbp | 78.9% (729) | 82.4% (699) | Not serious |
| NadA | 92.3% (725) | 95.3% (700) | |||||
| NHBA | 61.1% (731) | 69.5% (704) | |||||
| PorA | 42.4% (693) | 57.2% (664) | |||||
Appendix 4 Footnotes
Appendix 5: Summary of Studies Reporting Geometric Mean Titer (GMT)
| Intervention | Comparator | Author, year or Study ID | Population | Serogroup (test strain) |
Time since last study dose | GMT (N) Intervention | GMT (N) Comparator | Study limitations (risk of bias) |
|---|---|---|---|---|---|---|---|---|
| Short-term geometric mean hSBA titersa for serogroups A, C, W, and Y | ||||||||
| MenABCWY (1 dose) | MenACWY-CRM (1 dose) | Saez-Llorens 2015b | 11–18 years; ACWY naïve |
A | Baseline | 1.28 (82) | 1.4 (82) | |
| 1 month | 49 (79) | 105 (78) | ||||||
| C | Baseline | 4.57 (82) | 4.04 (81) | |||||
| 1 month | 76 (80) | 59 (79) | ||||||
| W | Baseline | 20 (82) | 19 (82) | |||||
| 1 month | 183 (79) | 188 (80) | ||||||
| Y | Baseline | 4.35 (82) | 5.76 (82) | |||||
| 1 month | 67 (79) | 77 (81) | ||||||
| Beran 2021c | 10–25 years; ACWY naïve |
A | Baseline | 3.19 (98) | 3.27 (97) | |||
| 1 month | 31.38 (98) | 52.03 (97) | ||||||
| C | Baseline | 5.12 (98) | 5.01 (97) | |||||
| 1 month | 41.35 (98) | 34.66 (97) | ||||||
| W | Baseline | 9.17 (98) | 16.11 (97) | |||||
| 1 month | 108.91 (98) | 80.11 (97) | ||||||
| Y | Baseline | 1.59 (98) | 1.84 (97) | |||||
| 1 month | 75.07 (98) | 92.41 (97) | ||||||
| MenACWY-CRM + MenB-4C administered in same arm (1 dose) | A | Baseline | 3.12 (101) | |||||
| 1 month | 67.95 (101) | |||||||
| C | Baseline | 4.62 (101) | ||||||
| 1 month | 33.76 (101) | |||||||
| W | Baseline | 11.69 (101) | ||||||
| 1 month | 81.38 (101) | |||||||
| Y | Baseline | 1.83 (101) | ||||||
| 1 month | 76.48 (101) | |||||||
| MenACWY-CRM + MenB-4C administered in different arms (1 dose) | A | Baseline | 3.21 (97) | |||||
| 1 month | 88.29 (97) | |||||||
| C | Baseline | 5.64 (97) | ||||||
| 1 month | 40.33 (97) | |||||||
| W | Baseline | 12.17 (97) | ||||||
| 1 month | 92.84 (97) | |||||||
| Y | Baseline | 2.05 (97) | ||||||
| 1 month | 100.30 (97) | |||||||
| MenACWY-CRM (1 dose) | V72_72d | 10–25 years; ACWY naïve or primed |
A | Baseline | 11.1 (1,452) | 12.7 (137) | ||
| 1 month | 175.3 (132) | 474.8 (133) | ||||||
| C | Baseline | 12.0 (1,487) | 11.4 (139) | |||||
| 1 month | 674.8 (139) | 379.0 (136) | ||||||
| W | Baseline | 8.0 (1,473) | 7.4 (140) | |||||
| 1 month | 374.0 (142) | 194.3 (137) | ||||||
| Y | Baseline | 9.3 (1,489) | 9.9 (141) | |||||
| 1 month | 375.4 (146) | 320.9 (140) | ||||||
| MenABCWY_019e | 15–25 years; ACWY primed |
A | Baseline | 15.30 (546) | 16.34 (549) | |||
| 1 month | 670.78 (605) | 1,282.56 (585) | ||||||
| C | Baseline | 31.90 (601) | 29.76 (584) | |||||
| 1 month | 2,945.68 (609) | 2,552.27 (593) | ||||||
| W | Baseline | 12.08 (597) | 11.08 (583) | |||||
| 1 month | 1,899.60 (607) | 1,665.64 (592) | ||||||
| Y | Baseline | 12.84 (600) | 11.86 (576) | |||||
| 1 month | 1,590.71 (606) | 1,578.42 (591) | ||||||
| Short-term geometric mean hSBA titersa for serogroup B | ||||||||
| MenABCWY (2 doses at 0,6 months) | MenB-4C (2 doses at 0,6 months) | V72_72d | 10–25 years; B naïve |
fHbp | Baseline | 2.8 (762) | 2.7 (730) | |
| 1 month | 25 (738) | 28.1 (707) | ||||||
| NadA | Baseline | 8.5 (780) | 8.3 (731) | |||||
| 1 month | 150.6 (734) | 215.1 (707) | ||||||
| NHBA | Baseline | 3.1 (764) | 3.2 (731) | |||||
| 1 month | 25.2 (738) | 33.2 (711) | ||||||
| PorA | Baseline | 3.1 (751) | 3.1 (716) | |||||
| 1 month | 12.9 (709) | 17.7 (684) | ||||||
| Persistent geometric mean hSBA titersa for serogroups A, C, W, and Y | ||||||||
| No relevant studies | ||||||||
| Persistent geometric mean hSBA titersa for serogroup B | ||||||||
| MenABCWY (2 doses at 0,6 months) | MenB-4C (2 doses at 0,2 months) | Vesikari 2021 | 10–18 years; B naïve | fHbp | Two years | 2.62 (70) | 2.04 (119) | |
| NadA | 16 (72) | 20 (121) | ||||||
| NHBA | 5.30 (71) | 4.24 (122) | ||||||
| PorA | 2.17 (71) | 1.72 (121) | ||||||
Appendix 5 Footnotes
- The EndNote Team. EndNote. Philadelphia, PA: Clarivate;
- Microsoft Corporation. Excel (Microsoft 365 Subscription). Microsoft Corporation; 2024.
- Saez-Llorens X, Aguilera Vaca DC, Abarca K, Maho E, Han L, Smolenov I, et al. Persistence of Meningococcal Antibodies and Response to a Third Dose After a Two-dose Vaccination Series with Investigational MenABCWY Vaccine Formulations in Adolescents. Pediatr Infect Dis J. 2015 Oct;34(10):e264-278.
- Sáez-Llorens X, Beltran-Rodriguez J, Novoa Pizarro JM, Mensi I, Keshavan P, Toneatto D. Four-year antibody persistence and response to a booster dose of a pentavalent MenABCWY vaccine administered to healthy adolescents and young adults. Hum Vaccin Immunother. 2018 May 9;14(5):1161–74.
- Szenborn L, Block SL, Jackowska T, Konior R, D’Agostino D, Smolenov I, et al. Immune Responses to Booster Vaccination With Meningococcal ABCWY Vaccine After Primary Vaccination With Either Investigational or Licensed Vaccines: A Phase 2 Randomized Study. The Pediatric Infectious Disease Journal. 2018 May;37(5):475.
- Saez-Llorens X, Aguilera Vaca DC, Abarca K, Maho E, Graña MG, Heijnen E, et al. Immunogenicity and safety of investigational vaccine formulations against meningococcal serogroups A, B, C, W, and Y in healthy adolescents. Hum Vaccin Immunother. 2015 May 13;11(6):1507–17.
- Block SL, Szenborn L, Daly W, Jackowska T, D’Agostino D, Han L, et al. A comparative evaluation of two investigational meningococcal ABCWY vaccine formulations: Results of a phase 2 randomized, controlled trial. Vaccine. 2015 May 15;33(21):2500–10.
- Welsch JA, Senders S, Essink B, Klein T, Smolenov I, Pedotti P, et al. Breadth of coverage against a panel of 110 invasive disease isolates, immunogenicity and safety for 2 and 3 doses of an investigational MenABCWY vaccine in US adolescents – Results from a randomized, controlled, observer-blind phase II study. Vaccine. 2018 Aug 23;36(35):5309–17.
- Vesikari T, Brzostek J, Ahonen A, Paassilta M, Majda-Stanislawska E, Szenborn L, et al. Immunogenicity and safety of different schedules of the meningococcal ABCWY vaccine, with assessment of long-term antibody persistence and booster responses – results from two phase 2b randomized trials in adolescents. Hum Vaccin Immunother. 17(11):4689–700.
- Beran J, Dražan D, Enweonye I, Bhusal C, Toneatto D. Immunogenicity and Safety of Investigational MenABCWY Vaccine and of 4CMenB and MenACWY Vaccines Administered Concomitantly or Alone: a Phase 2 Randomized Study of Adolescents and Young Adults. mSphere. 6(6):e00553-21.
- GSK. A Phase III, Randomized, Controlled, Observer-blind Study to Demonstrate Effectiveness, Immunogenicity and Safety of GSK's Meningococcal Group B and Combined ABCWY Vaccines When Administered to Healthy Adolescents and Young Adults [Internet]. clinicaltrials.gov; 2024 Feb [cited 2025 Feb 4]. Report No.: NCT04502693. Available from: https://clinicaltrials.gov/study/NCT04502693
- GSK. A Phase IIIB, Randomized, Controlled, Observer-blind Study to Evaluate Safety and Immunogenicity of GSK's Meningococcal ABCWY Vaccine When Administered in Healthy Adolescents and Adults, Previously Primed With Meningococcal ACWY Vaccine [Internet]. clinicaltrials.gov; 2024 June [cited 2025 Feb 5]. Report No.: NCT04707391. Available from: https://clinicaltrials.gov/study/NCT04707391
