About
CDC vaccine recommendations are developed using an explicit evidence-based method based on the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Introduction
On February 26, 2020, the Advisory Committee on Immunization Practices (ACIP) recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP vaccine for adults aged 18 years or older in the United States population who are at potential risk of exposure to EBOV:
- Persons who are responding to an outbreak of EVD
- Persons who work as healthcare personnel (HCP)* at federally designated Ebola Treatment Centers in the United States
- Persons who work as laboratorians or other staff at biosafety-level 4 facilities in the United States
Previously, ACIP had no recommendations for the use of vaccines to prevent EVD. Beginning in September 2019, the ACIP Ebola Vaccine Work Group (WG) met regularly to define the research questions of interest, identify critical and important patient-centered outcomes, conduct a systematic review of the evidence, assess the certainty of the evidence, and make recommendations according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Methods and GRADE
A systematic review and assessment of the evidence for the rVSVΔG-ZEBOV-GP Ebola vaccine was conducted and presented to ACIP in February 2020. No conflicts of interest were reported by CDC staff or the WG members involved in the review. As a basis for the systematic review, the policy question consisting of the population, intervention, comparison, and outcomes (PICO) of interest was defined according to the GRADE approach (Table 1). For each PICO question, desirable and undesirable patient-important outcomes were selected by the WG. Outcomes deemed “Critical” and “Important” by the WG are presented in Table 1. All critical outcomes were included in the evidence profiles unless otherwise indicated in Table 1. Evidence included in the profiles is the same across the three population categories.
On December 16, 2019, a literature search was executed in multiple biomedical and interdisciplinary bibliographic databases using a broad and rigorous search strategy that incorporated terms related to vaccination against Ebola virus using the rVSVΔG-ZEBOV-GP vaccine, without date or language restrictions. Results were compiled in an Endnote library and duplicate records were removed. The search was updated on January 31, 2020 to screen recent records that were not captured in the original search. Efforts were made to obtain unpublished or other relevant data not included in the search results from subject matter experts and the manufacturer and yielded one additional record for inclusion in analysis.
Records were included for analysis if they presented data on the rVSVΔG-ZEBOV-GP Ebola vaccine and involved immunocompetent adults 18 years of age or older, regardless of pregnancy status, included data for the intervention of interest and data relevant to the outcome measures being assessed; and reported primary data from comparative or single-arm studies; randomized control trials, prospective or retrospective cohort, case-control, or cross-sectional studies. A total of 1,818 records were identified through database searches and one unpublished record was identified through other sources. A total of 1,742 of these records were excluded during title and abstract screening, leaving 77 full-text articles that were assessed for eligibility through full-text review. Of these, 59 full-text articles were excluded (Figure 1). In total, 18 articles that presented data from 11 studies were included in qualitative synthesis while 9 articles that presented data from 8 studies were included in quantitative synthesis or meta-analysis (Figure 1).
The GRADE approach for assessing the type or quality of evidence involves consideration of several criteria. Assessing the type or certainty level of the body of evidence for each outcome begins with the study design. Randomized control trials are initially classified as evidence type 1, high certainty, and observational studies as evidence type 3, low certainty. Following the identification of the initial evidence type, the body of evidence for each outcome is assessed and downgraded if there is uncertainty about any of the five following criteria: risk of bias; inconsistency, which considers statistical heterogeneity and I2, defined as the percent variation across studies due to heterogeneity instead of chance; indirectness, which is the generalizability of the body of evidence to the original PICO components; imprecision, which considers the fragility of the relative and absolute effect measures as they relate to the 95% confidence intervals and optimal information size; and publication bias. The body of evidence from observational studies may be rated up due to dose-response gradient, large or very large magnitude of effect, or opposing residual confounding.
After assessing on these criteria, the body of evidence was assigned an overall evidence type or certainty level. Type 1, high certainty, evidence can be interpreted that there is a high level of confidence that the true effect lies close to that of the estimate of effect. Type 2, moderate certainty, evidence can be interpreted that there is a moderate level of confidence in the effect estimate. Type 3, low certainty, evidence can be interpreted that confidence in the effect estimate is limited. Type 4, very low certainty, evidence can be interpreted that there is little confidence in the effect estimate. This does not measure how well the individual studies were conducted, but rather how much confidence there is in the estimates of effect from the body of evidence across each outcome.
For the purposes of the evidence assessment and evidence profile tables, randomized control trial (RCT) refers to a trial which randomizes participants to an active intervention or a placebo or unvaccinated comparator arm; observational studies refer to one-arm studies, studies for whom participants were not randomized, or studies that did not provide disaggregated data to allow for the comparison between the randomized arms. Evidence was also considered observational if only data from the vaccinated study arms were included in analysis for a given outcome. Observational studies without comparators are not included in the analyzed bodies of evidence but would be evidence type 4, very low certainty.
Summary of Findings
Development of Ebola-related symptomatic illness
There was one published study with an unvaccinated comparator that was included for the body of evidence for this outcome, the publication of the final results associated with the Ça Suffit! Trial in Guinea1. This was a two-part phase 3 cluster-randomized open-label ring vaccination trial1. The initial findings from this trial were published in an interim report2. Only data from the final report 1was included in the analyses to prevent duplication. The initial study involved contacts and contacts of contacts of confirmed Ebola virus disease or EVD cases that were randomized to either immediate or delayed vaccination. Delayed vaccination was defined as vaccination that occurred 21 days after randomization. A follow-up study included immediate vaccination following cessation of the randomized trial. The primary outcome was the incidence of EVD with onset of 10 days or more following randomization. The 10 days accounts for the average incubation period of Ebola and unknown time for the vaccine to induce protective immunity.
Cluster- and participant-level data from randomized clusters are presented in the evidence table for this outcome in Table 2. Out of 3775 participants within 70 clusters that received immediate vaccination (between randomized and non-randomized), 0 participants developed EVD 10 days or longer after randomization1. In contrast, of 4507 participants within 104 clusters that were delayed or never received the vaccine, 23 participants within 11 clusters developed EVD 10 days or longer after randomization1. Within randomized clusters, out of 2108 participants within 51 clusters that received immediate vaccination, 0 developed EVD greater than 10 days after randomization1. In contrast, of 3075 participants within 47 clusters that were randomized to delayed vaccination, 16 participants within 7 clusters developed EVD greater than 10 days after randomization. These randomized data equate to a calculated vaccine efficacy of 100% (95% confidence interval 68.9 – 100).1
Given that this was a cluster randomized trial where the units of randomization were clusters, the randomized cluster-level evidence is presented in Table 2. Because the population in this study consists of contacts and contacts of contacts of EVD cases and used a ring vaccination strategy that may include post-exposure vaccination, the certainty level was downgraded one level for indirectness to the U.S. population and the intervention of interest, which is pre-exposure vaccination. The cluster-level evidence was also downgraded one level for imprecision because there were few events reported and the data do not meet the optimal information size and suggest fragility of the estimate, and the confidence interval crosses 1 and contains the potential for desirable as well as undesirable effects. Considering this assessment, the overall assessment of this body of evidence at the randomized cluster level to address the outcome of development of Ebola-related symptomatic illness is type 3, low certainty evidence (Table 2).
Participant-level data from the randomized clusters is also presented in Table 2 and was considered observational because the units of randomization within the study were clusters. Because of the very precise reduction in risk for those immediately vaccinated, the body of evidence was not rated down for imprecision at the participant level. The evidence was also rated down one level for indirectness for the same reason as the cluster-level data, however, the concerns with indirectness did not pose an inflationary effect; and therefore, the evidence could be rated up based on a very large magnitude of effect from the 96% reduction in risk for those immediately vaccinated. Taken together, overall certainty was upgraded two levels to type 2, moderate certainty evidence for this participant level data (Table 2).
Incidence of arthralgia
The outcome of incidence of arthralgia was assessed with the incidence of arthralgia or joint pain that was solicited within 0-42 days. The body of evidence of RCTs and observational studies analyzed for this outcome are presented in Tables 3a and 3b, respectively. The body of evidence that included six RCTs345678 was downgraded one level for a concern for risk of bias because of lack of blinding in participants, healthcare personnel, and outcome assessors in two studies that may have influenced events reported for this outcome. Additionally, there is a concern for underreporting in one study that only solicited arthralgia at one week and one month for the majority of participants, that may have led to underreporting of events. Due to concerns with heterogeneity with an I2 of 70%, this study was downgraded one level for inconsistency. Because the 95% confidence interval crosses 1 and includes a potential for possible harms as well as benefits, it was downgraded one level for imprecision. Overall, this body of evidence was assessed to be type 4, very low certainty evidence (Table 4). The body of evidence from the two observational studies 59was downgraded one level for imprecision because there were few events reported suggesting fragility in the estimate. Overall, this body of evidence was assessed to be type 4, very low certainty evidence (Table 4).
Severity of arthralgia
The outcome of severity of arthralgia was assessed with the incidence of severe (grade 3) arthralgia solicited between 0-42 days and defined as significant joint pain or discomfort that prevents daily activity. The body of evidence of RCTs and observational studies analyzed for this outcome are presented in Tables 5a and 5b, respectively. The body of evidence that included four RCTs 3578was downgraded one level for a concern for risk of bias because of lack of blinding in participants, healthcare personnel, and outcome assessors in two studies that may have influenced events reported for this outcome. Because of there being a concern for fragility in the estimate due to the few numbers of events reported, it was downgraded one level for imprecision. Overall, this body of evidence was assessed to be type 3, low certainty evidence (Table 6). The body of evidence for the two observational studies 59was downgraded one level for imprecision because there were no events reported among vaccinated or non-vaccinated participants and it suggests fragility in the estimate. Overall, this body of evidence was assessed to be type 4, very low certainty evidence (Table 6).
Incidence of arthritis
The outcome of incidence of arthritis was assessed with an event of arthritis reported within 5-56 days of follow up. The body of evidence of RCTs and observational studies analyzed for this outcome are presented in Tables 7a and 7b, respectively. The body of evidence that included four randomized trials3467 was downgraded one level for a concern for risk of bias because studies used variable definitions and methods for diagnosing and reporting arthritis, and for lack of blinding in participants, healthcare personnel, and outcome assessors in two studies that may have influenced events reported for this outcome. Because the 95% confidence interval crosses 1 and includes a potential for possible harms as well as benefits, we downgraded one level for imprecision. Overall, this body of evidence was assessed to be type 3 low certainty evidence (Table 8). The body of evidence for the two observational studies 59was downgraded two levels for imprecision because of a concern for fragility in the estimate due to the few number of events reported and that the 95% confidence interval crosses 1 and includes a potential for possible harms as well as benefits. Overall, this body of evidence was assessed to be type 4 very low certainty evidence (Table 8).
Vaccine-related adverse pregnancy outcomes for women inadvertently vaccinated while pregnant and women who become pregnant within in 2 months of vaccination
This outcome was assessed with incidence of pregnancy loss (defined as spontaneous abortion and stillbirth). There was one study included in the body of evidence for this outcome, a non-randomized sub-study of the Sierra Leone Trial to Introduce a Vaccine Against Ebola.10 The body of evidence for outcome was downgraded one level for indirectness because the study did not differentiate between spontaneous abortions (including induced abortion) and stillbirth, and the outcome may not accurately distinguish between events due to the vaccine. Additionally, because of there being a concern for fragility in the estimate due to the few numbers of events reported and that the 95% confidence interval crosses 1 and includes a potential for possible harms as well as benefits, it was downgraded two levels for imprecision. Overall, this body of evidence was assessed to be type 4 very low certainty evidence (Table 9). There were 3 additional studies 41112that reported on this outcome that did not have comparators. Among these 3 studies, there were 3 adverse pregnancy outcomes out of 20 pregnancies in 19 women; however, no conclusions can be made regarding the relationship between vaccination and adverse pregnancy outcomes based on these data.
Transmissibility of rVSVΔG-ZEBOV-GP vaccine virus to humans or animals
rVSVΔG-ZEBOV-GP vaccine virus dissemination was used as a surrogate for this outcome due to lack of available data on transmissibility and was assessed with detection of rVSVΔG-ZEBOV-GP in blood/plasma, saliva, and urine by RT-PCR. Data from 8 studies 35689111314were included for the body of evidence for the surrogate outcome of detection of rVSVΔG-ZEBOV-GP in blood/plasma by RT-PCR. Across these 8 studies, on day 7 post-vaccination, 32 out of 691 vaccinated participants (or 4.6%) were RT-PCR positive for vaccine virus. On day 14 post-vaccination, 1 out of 501 (or 0.2%) of vaccinated participants were RT-PCR positive for vaccine virus; however, true estimates of duration of viremia is unknown because daily collection was not performed. Additionally, one study performed viral isolation on selected blood specimens, and all were negative. Data from 4 studies 38913were included for the body of evidence for the surrogate outcome of detection of rVSVΔG-ZEBOV-GP in saliva and urine by RT-PCR. Across these 4 studies, on day 7 post-vaccination, 6 out of 257 vaccinated participants (or 2.3%) were RT-PCR positive for vaccine virus in saliva while 2 out of 246 (0.8%) were positive in urine. On day 14 post-vaccination, 1 out of 98 (or 1%) of vaccinated participants were positive in saliva while 0/98 were positive in urine.
For the purposes of this outcome, only data from the vaccinated arms were included for analysis, so all included studies were considered observational. The body of evidence was downgraded one level for risk of bias because of concern for incomplete outcome data as not all who received the vaccine were tested on a given day. It was also downgraded 2 levels for indirectness because the outcome of interest to the work group was transmissibility of the vaccine virus to humans or animals. There are no data that report on transmissibility, so viral dissemination and shedding is assessed as an indirect surrogate. Additionally, RT-PCR positivity is not synonymous with infectivity. Overall, the body of evidence for both the surrogate outcomes of detection of rVSVΔG-ZEBOV-GP in blood/plasma and detection of rVSVΔG-ZEBOV-GP in saliva and urine by RT-PCR was assessed to be type 4 very low certainty evidence (Table 10a and 10b, respectively).
Vaccine-related serious adverse events
Vaccine-related serious adverse events following vaccination with rVSVΔG-ZEBOV-GP are an uncommon occurrence. Across the body of evidence from 12 clinical trials and two additional publications that describe use of vaccine through expanded use mechanisms that reported on vaccine-related serious adverse events 345789111314151617(0.02%) vaccinees were judged to have a SAE related to or possibly related to vaccination. Two of the three were related to vaccination and included a febrile reaction and anaphylaxis, both of which resolved without sequelae.1 One was judged to be possibly related to the vaccine, an influenza like illness, which also resolved without sequelae.1 An additional publication 18presented serious adverse events captured during the Sierra Leone Trial to Introduce a Vaccine Against Ebola; however, overall safety findings from this trial were previously reported and included in this analysis 7so the additional publication was not included. Like the previous outcome, only data from the vaccinated arms were included for analysis, so the studies were considered observational for this outcome and we did not downgrade across any of the criteria. Overall, this body of evidence was assessed to be type 3 low certainty evidence.
GRADE Analysis for VSVΔG-ZEBOV-GP Ebola vaccine for persons in the U.S. population at potential occupational risk of exposure to Ebola virus
Figure 1:
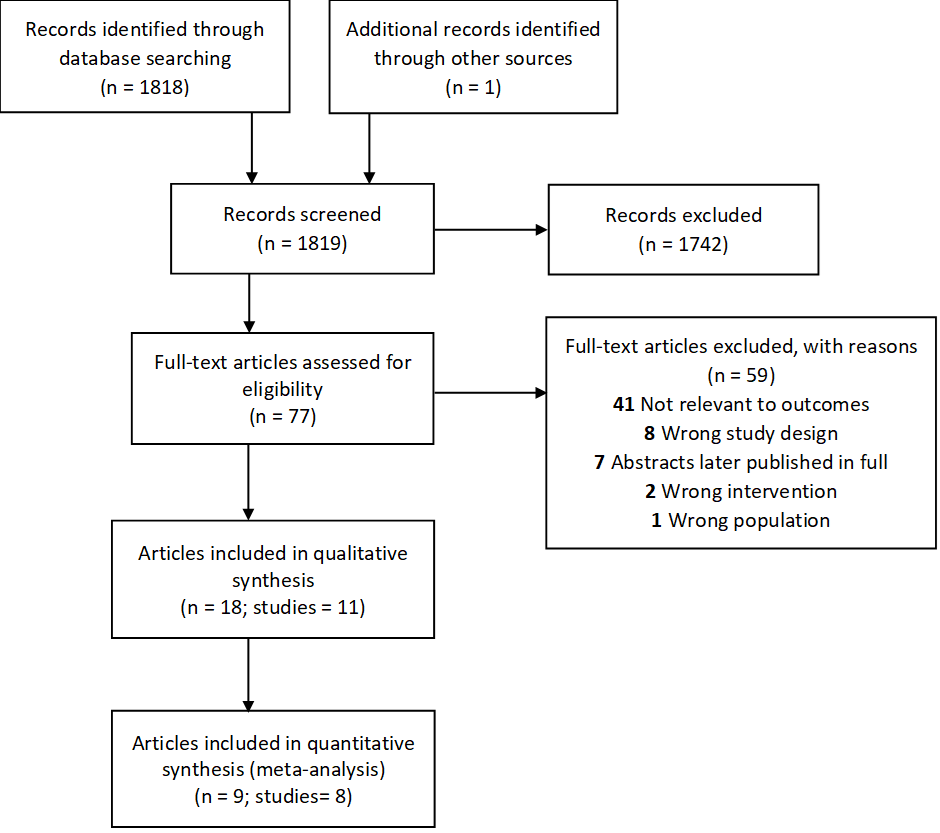
Table 1: Policy question and definition of the Population, Intervention, Comparison, and Outcomes determined by the ACIP Ebola Vaccine Work Group for GRADE analysis.
| Policy question: Should pre-exposure vaccination with the rVSVΔG-ZEBOV-GP vaccine be recommended for adults 18 years of age or older in the U.S. population who are at potential occupational risk of exposure to Ebola virus (species Zaire ebolavirus) for prevention of Ebola virus infection? | |
|---|---|
| Population | Adults aged 18 years or older in the United States population who are at potential risk of exposure to EBOV because they are:
|
| Intervention | Pre-exposure intramuscular immunization with a single licensed dose of the rVSVΔG-ZEBOV-GP vaccine |
| Comparison | No vaccine |
| Outcomes | Benefits:
Harms:
|
Bold font indicates outcomes that were deemed “Critical” by the Work Group and included for GRADE analysis
† Deemed “Critical”; however, was not included for analysis as there was no available data for this outcome
Table 2. Evidence table for outcome of development of Ebola-related symptomatic illness
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | rVSV- vaccine | no rVSV- vaccine | Relative (95% CI) |
Absolute (95% CI) |
||
|
11 |
Randomizeda |
not serious |
not serious |
seriousb |
seriousc |
none |
0/51 (0.0%) |
7/47 (14.9%) |
RR 0.06g |
140 fewer per 1,000 |
LOW |
CRITICAL |
|
11 |
Observationald |
not serious |
not serious |
seriousb |
not serious |
strong associatione |
0/2108f (0.0%) |
16/3075 (0.5%) |
RR 0.04g |
5 fewer per 1,000 (from 5 fewer to 1 fewer |
MODERATEe |
CRITICAL |
Note: Outcome assessed with laboratory confirmed case of EVD
Explanations
a. Henao-Restrepo 2017 was a cluster randomized trial (i.e. units of randomization were clusters); cluster-level data presented here
b. Concern for indirectness to US population: population consists of contacts and contacts of contacts of EVD case, ring vaccination strategy which may include post-exposure vaccination
c. Because this study was done at a time when the 2014-2015 West Africa outbreak was waning in Guinea and there are few events reported, it does not meet optimal information size and suggests fragility in the estimate; 95% CI contains the potential for desirable as well as undesirable effects
d. Henao-Restrepo 2017 was a cluster randomized trial (i.e. units of randomization were clusters); participant-level data presented here
e. The concerns with indirectness pose no inflationary effect; therefore, the evidence was rated up based on a very large magnitude of effect from the 96% reduction in risk and overall certainty was upgraded two levels
f. Denominator represents participants from the clusters randomized to receive immediate vaccination
g. RR calculated using the standard continuity correction of 0.5
CI: Confidence interval; RR: Relative risk
Table 3a. Estimates of effect for RCTs included in analysis for outcome of incidence of arthralgia (0-42 days)
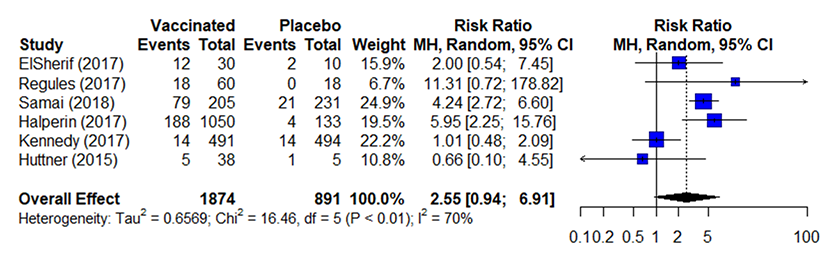
Table 3b. Estimates of effect for observational studies included in analysis for outcome of incidence of arthralgia (0-42 days)
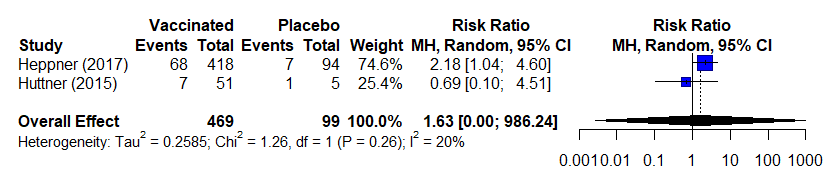
Notes:
- Studies used variable definitions for arthralgia or in some cases a definition was not provided.
- Concern for underreporting because length and time of follow up/solicitation varied between studies; however, did not have an impact on effect estimates for this analysis.
- Data presented from across several doses of vaccine (strengths /varying PFUs); however, there does not seem to be a dose-response or effect on this outcome
- Huttner 2015 did not solicit arthralgia for high-dose participants; these data were excluded from analysis
- RR calculated using the standard continuity correction of 0.5 and the overall effect uses a random effects model
- Across 7 studies 1111213141516that included data without comparators, 1,546 out of 8,329 (16%) of vaccinated participants reported arthralgia
RR, risk ratio; CI, confidence interval; MH, Mantel-Haenszel; df, degree of freedom; I2 , % of variation across studies due to heterogeneity
Table 4. Evidence table for outcome of incidence of arthralgia (0-42 days).
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | rVSV-ZEBOV vaccine | no rVSV-ZEBOV vaccine | Relative (95% CI) |
Absolute (95% CI) |
||
|
63,4,5,6,7,8 |
Randomized trials |
seriousa |
seriousb |
not serious |
seriousc |
none |
316/1874 (16.9%) |
42/891 (4.7%) |
RR 2.55e (0.94 to 6.91) |
73 more per 1,000 (from 3 fewer to 279 more) |
VERY LOW |
CRITICAL |
|
25,9 |
Observational studies |
not serious |
not serious |
not serious |
seriousd |
none |
75/469 (16.0%) |
8/99 (8.1%) |
RR 1.63e (0 to 986.24) |
51 more per 1,000 (from 81 fewer to 1,000 more) |
VERY LOW |
CRITICAL |
Explanations
Note: Observational studies without comparators are not included in evidence table, but would be considered of very low certainty (evidence type4)
a. Participants, healthcare personnel, and outcome assessors were not blinded in Huttner 2015 or Samai 2018 potentially influencing events reported for this subjective outcome. Concern for possible underreporting in Kennedy because arthralgia was only solicited at one week and at one month for most participants; Huttner only solicited arthralgia for low dose participants
b. Concerns with heterogeneity (I2 =70%) some may be explained by concerns with risk of bias (poor randomization or outcome definition)
c. The 95% confidence interval includes potential for possible harms as well as benefits
d. Few events reported do not meet optimal information size and suggest fragility in the estimate
e. RR calculated using the standard continuity correction of 0.5 and uses a random effects model
CI: Confidence interval; RR: Relative risk
Table 5a. Estimates of effect for RCTs included in analysis for outcome of incidence of severe (grade 3) arthralgia (0-42 days).
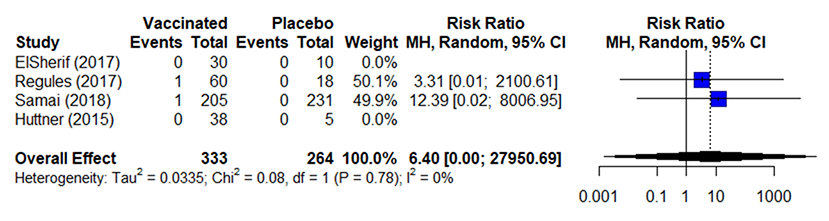
Table 5b. Estimates of effect for observational studies included in analysis for outcome of incidence of severe (grade 3) arthralgia (0-42 days).
Notes:
- Studies used variable definitions for arthralgia or in some cases a definition was not provided.
- Concern for underreporting because length and time of follow up/solicitation varied between studies; however, did not have an impact on effect estimates for this analysis. There is a concern that pooling these data may under-estimate incidence because of this variability
- Data presented from across several doses of vaccine (strengths /varying PFUs); however, there does not seem to be a dose-response or effect on this outcome
- Huttner 2015 did not solicit arthralgia for high-dose participants; these data were excluded from analysis
- Risk ratios (RR) were calculated using a 0.1 continuity correction due to low numbers of reported events and the overall effect uses a random effects model
- Across 5 studies 111131416that included data without comparators, 7/7,209 (0.1%) vaccinated participants reported severe (grade 3) arthralgia
RR, risk ratio; CI, confidence interval; MH, Mantel-Haenszel; df, degree of freedom; I2, % of variation across studies due to heterogeneity
Table 6. Evidence table for outcome of incidence of severe (grade 3) arthralgia (0-42 days).
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | rVSV-ZEBOV vaccine | no rVSV-ZEBOV vaccine | Relative (95% CI) |
Absolute (95% CI) |
||
|
43,5,7,8 |
Randomized trials |
seriousa |
not serious |
not serious |
seriousb |
none |
2/333 (0.6%) |
0/264 (0.0%) |
RR 6.40c (0 to 27950.69) |
0 fewer per 1,000 (from 0 fewer to 0 fewer) |
LOW |
CRITICAL |
|
23,9 |
Observational studies |
not serious |
not serious |
not serious |
seriousb |
none |
No events of grade 3 arthralgia were reported among 469 vaccinated and 99 non-vaccinated participantsd |
VERY LOW |
CRITICAL |
|||
Note: Observational studies without comparators are not included in evidence table, but would be considered of very low certainty (evidence type 4)
Explanations
- Participants, healthcare personnel, and outcome assessors were not blinded in Huttner 2015 or Samai 2018 potentially influenced events reported for this subjective outcome. Huttner only solicited arthralgia for low dose participants
- Few events reported do not meet optimal information size and suggest fragility in the estimate
- Risk ratios (RR) were calculated using a 0.1 continuity correction due to low numbers of reported events and the overall effect uses a random effects model
- Huttner 2015 did not solicit arthralgia for high-dose participants; these data were excluded from analysis
CI: Confidence interval; RR: Relative risk
Table 7a. Estimates of effect for RCTs included in analysis for outcome of incidence of arthritis (5-56 days).
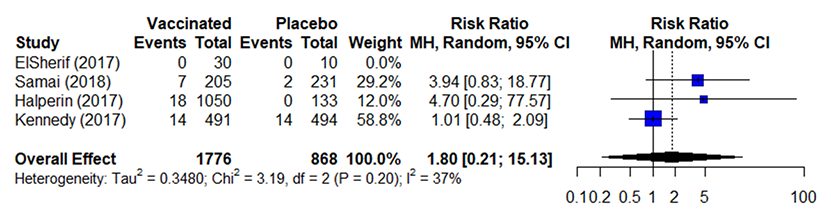
Table 7b. Estimates of effect for observational studies included in analysis for outcome of incidence of arthritis (5-56 days).
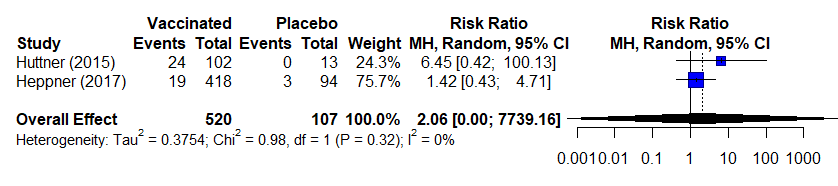
Notes:
- Studies defined and worked up arthritis with considerable variability.
- Kennedy 2017: Concern for underreporting because low % of female enrolled participants (37%); only solicited at week 1 and at month 1
- Samai 2018: No capability of clinical diagnosis of arthritis, no rheumatology services available in Sierra Leone
- ElSherif 2017: Did not provide definition for arthritis
- Huttner 2015: First to encounter arthritis, so thoroughly clinically investigated arthritis (all participants with arthritis referred to rheumatologist, all but 2 participants with arthritis had an u/s done); this study not included in RCT analysis because arthritis only reported in low dose participants and upon request de-aggregated data was unavailable
- Kennedy 2017: Concern for underreporting because low % of female enrolled participants (37%); only solicited at week 1 and at month 1
- Concern for underreporting because length and time of follow up/solicitation varied between studies; however, did not have an impact on effect estimates for this analysis. There is a concern that pooling these data may under-estimate incidence because of this variability
- Data presented from across several doses of vaccine (strengths /varying PFUs); however, there does not seem to be a dose-response or effect on this outcome
- RR calculated using the standard continuity correction of 0.5 and the overall effect uses a random effects model
- Across 2 studies 1314that included data without comparators, 2/50 (4%) vaccinated participants reported arthritis
RR, risk ratio; CI, confidence interval; MH, Mantel-Haenszel; df, degree of freedom; I2, % of variation across studies due to heterogeneity
Table 8. Evidence table for outcome of incidence of arthritis (5-56 days).
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | rVSV-ZEBOV vaccine | no rVSV-ZEBOV vaccine | Relative (95% CI) |
Absolute (95% CI) |
||
|
43,4,6,7 |
Randomized trials |
seriousa |
not serious |
not serious |
seriousb |
none |
39/1776 (2.2%) |
16/868 (1.8%) |
RR 1.80d (0.21 to 15.13) |
23 more per 1,000 (from 22 fewer to 400 more) |
LOW |
CRITICAL |
|
23,9 |
Observational studies |
not serious |
not serious |
not serious |
very seriousb,c |
none |
43/520 (8.3%) |
3/107 (2.8%) |
RR 2.06d (0.0001 to 7739.16) |
33 more per 1,000 (from 28 fewer to 1,000 more) |
VERY LOW |
CRITICAL |
Note: Observational studies without comparators are not included in evidence table, but would be considered of very low certainty (evidence type 4)
Explanations
a. Studies used variable definitions and methods for diagnosing and reporting arthritis. In addition, participants, healthcare personnel, and outcome assessors were not blinded in Huttner 2015 or Samai 2018 potentially influencing events reported for this subjective outcome.
b. The 95% CI includes the potential for possible harms, as well as possible benefit.
c. Few events reported do not meet optimal information size and suggest fragility in the estimate.
d. RR calculated using the standard continuity correction of 0.5 and the overall effect uses a random effects model.
CI: Confidence interval; RR: Relative risk
Table 9. Evidence table for outcome of vaccine-related adverse pregnancy outcomes for women inadvertently vaccinated while pregnant and women who become pregnant within in 2 months of vaccination.
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | rVSV-ZEBOV vaccine | no rVSV-ZEBOV vaccine | Relative (95% CI) |
Absolute (95% CI) |
||
|
110 |
Observational |
not seriousa |
not serious |
seriousb |
very seriousc,d |
none |
14/31 (45.2%) |
11/33 (33.3%) |
RR 1.35 (0.73 to 2.52) |
117 more per 1,000 (from 90 fewer to 507 more) |
VERY LOW |
CRITICAL |
Note: Observational studies without comparators are not included in evidence table, but would be considered of very low certainty (evidence type4)
Explanations
a. Participants, study personnel, and outcome assessors were unblinded and could have potentially influenced risk behaviors, though likely did not have an impact on risk of bias
b. Legardy-Williams et al. report on the outcome of pregnancy loss as a measure of vaccine-related pregnancy adverse events; however, the study did not differentiate between spontaneous abortions (which includes induced abortion) and stillbirths. The outcome may not accurately distinguish between those events due to the vaccine. In addition, we are not certain about the events reported that are directly related to receipt of the vaccine.
c. The 95% CI includes the potential for possible harms, as well as possible benefit.
d. Few events reported do not meet optimal information size and suggest fragility in the estimate.
CI: Confidence interval; RR: Relative risk
Table 10a. Evidence table for surrogate outcome of vaccine virus dissemination (assessed by detection of vaccine in blood/plasma by RT-PCR).
| Certainty assessment | Summary of Findings | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
|
83,5,6,8,9,11,13,14 |
Observational studiesa |
seriousb |
not serious |
very seriousc |
not serious |
none |
Longest recorded positive RT-PCR in blood or plasma is 14 days post-vaccination; 26/691 (3.7%) positive at day 7; 1/501 (0.2%) vaccinees positive at day 14. |
VERY LOW |
CRITICAL |
Table 10b. Evidence table for surrogate outcome vaccine virus dissemination (assessed by detection of vaccine in saliva and urine by RT-PCR).
| Certainty assessment | Summary of Findings | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
|
43,8,9,13 |
Observational studiesa |
seriousd |
not serious |
very seriousc |
not serious |
none |
Longest recorded positive RT-PCR in saliva is 14 days post-vaccination; 6/257 (2.3%) positive at day 7; 1/98 (1.0%) vaccinees positive at day 14. Longest recorded positive RT-PCR in urine is 7 days post-vaccination; 2/246 (0.8%) positive at day 7; 0/98 positive at day 14. |
VERY LOW |
CRITICAL |
Explanations
a. Outcome data was only collected from the vaccinated study arm from these studies; therefore, they were considered observational for these outcomes
b. Not all who received the vaccine were tested; concern for incomplete outcome data. Heppner 2017: 46/60 were tested on day 3, 49/60 were tested on day 7, and 30/60 were tested on day 14.
c. The outcome of interest is transmissibility of the vaccine virus to humans or animals. No data is available for, so viral dissemination and shedding is assessed as an indirect surrogate. RT-PCR positivity is not synonymous with infectivity.
d. Not all who received the vaccine were tested; concern for incomplete outcome data. ElSherif: Virus in urine and saliva were only tested if viremia was detected at or above the level of quantification; Heppner 2017: 46/60 were tested on day 3, 49/60 were tested on day 7, and 30/60 were tested on day 14.
CI: Confidence interval; RR: Relative risk
Table 11. Evidence table for surrogate outcome of vaccine-related serious adverse events.
| Certainty assessment | Summary of Findings | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
|
12 |
Observational studiesa |
not serious |
not serious |
not serious |
not serious |
none |
Across 12 studies, 3/17,119 (0.02%) vaccinees were judged to have an SAE related to or possibly related to vaccination. |
LOW |
CRITICAL |
Explanations
a. Outcome data was only collected from the vaccinated study arm from these studies; therefore, they were considered observational for these outcomes
b. Overall evidence type is 3 (low certainty) because these 12 studies were considered observational for these outcomes as data was only collected from the vaccinated study arm from these studies without a comparator; however there was no downgrading of the evidence.
CI: Confidence interval; RR: Relative risk
Footnote
*Healthcare personnel (HCP) refers to all paid and unpaid persons serving in healthcare settings who have the potential for direct or indirect exposure to patients or infectious materials, including body substances (e.g., blood, tissue, and specific body fluids); contaminated medical supplies, devices, and equipment; contaminated environmental surfaces; or contaminated air. These HCP include, but are not limited to, emergency medical service personnel, nurses, nursing assistants, physicians, technicians, clinical laboratory personnel, autopsy personnel, therapists, phlebotomists, pharmacists, students and trainees, contractual staff not employed by the healthcare facility, and persons not directly involved in patient care, but who could be exposed to infectious agents that can be transmitted in the healthcare setting (e.g., clerical, dietary, environmental services, laundry, security, engineering and facilities management, administrative, billing, and volunteer personnel). Adapted from https://www.cdc.gov/infection-control/about/index.html?CDC_AAref_Val=https://www.cdc.gov/infectioncontrol/guidelines/healthcare-personnel/index.html
- Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017;389:505-18.
- Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015;386:857-66.
- ElSherif MS, Brown C, MacKinnon-Cameron D, et al. Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. CMAJ 2017;189:E819-E27.
- Halperin SA, Arribas JR, Rupp R, et al. Six-Month Safety Data of Recombinant Vesicular Stomatitis Virus-Zaire Ebola Virus Envelope Glycoprotein Vaccine in a Phase 3 Double-Blind, Placebo-Controlled Randomized Study in Healthy Adults. J Infect Dis 2017;215:1789-98.
- Huttner A, Dayer JA, Yerly S, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015;15:1156-66.
- Kennedy SB, Bolay F, Kieh M, et al. Phase 2 Placebo-Controlled Trial of Two Vaccines to Prevent Ebola in Liberia. N Engl J Med 2017;377:1438-47.
- Samai M, Seward JF, Goldstein ST, et al. The Sierra Leone Trial to Introduce a Vaccine Against Ebola: An Evaluation of rVSVG-ZEBOV-GP Vaccine Tolerability and Safety During the West Africa Ebola Outbreak. J Infect Dis 2018;217:S6-S15.
- Regules JA, Beigel JH, Paolino KM, et al. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine. N Engl J Med 2017;376:330-41.
- Heppner DG, Jr., Kemp TL, Martin BK, et al. Safety and immunogenicity of the rVSVG-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect Dis 2017;17:854-66.
- Legardy-Williams JK, Carter RJ, Goldstein ST, et al. Pregnancy Outcomes among Women Receiving rVSVDelta-ZEBOV-GP Ebola Vaccine during the Sierra Leone Trial to Introduce a Vaccine against Ebola. Emerg Infect Dis 2020;26:541-8.
- Agnandji ST, Fernandes JF, Bache EB, et al. Safety and immunogenicity of rVSVDeltaG-ZEBOV-GP Ebola vaccine in adults and children in Lambarene, Gabon: A phase I randomised trial. PLoS Med 2017;14:e1002402.
- Juan-Giner A, Tchaton M, Jemmy JP, et al. Safety of the rVSV ZEBOV vaccine against Ebola Zaire among frontline workers in Guinea. Vaccine 2019;37:7171-7.
- Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe. N Engl J Med 2016;374:1647-60.
- Dahlke C, Kasonta R, Lunemann S, et al. Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization. EBioMedicine 2017;19:107-18.
- Bolay FK, Grandits G, Lane HC, et al. PREVAIL I Cluster Vaccination Study With rVSVDeltaG-ZEBOV-GP as Part of a Public Health Response in Liberia. J Infect Dis 2019;219:1634-41.
- Gsell PS, Camacho A, Kucharski AJ, et al. Ring vaccination with rVSV-ZEBOV under expanded access in response to an outbreak of Ebola virus disease in Guinea, 2016: an operational and vaccine safety report. Lancet Infect Dis 2017;17:1276-84.
- Halperin SA, Das R, Onorato MT, et al. Immunogenicity, Lot Consistency, and Extended Safety of rVSVDeltaG-ZEBOV-GP Vaccine: A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study in Healthy Adults. J Infect Dis 2019;220:1127-35.
- Jarrett OD, Seward JF, Fombah AE, et al. Monitoring Serious Adverse Events in the Sierra Leone Trial to Introduce a Vaccine Against Ebola. J Infect Dis 2018;217:S24-S32.
