 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Malaria Surveillance --- United States, 1997John R. MacArthur, M.D., M.P.H.1,2 1Epidemic Intelligence Service, Epidemiology Program Office AbstractProblem/Condition: Malaria is caused by four species of intraerythrocytic protozoa of the genus Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, or P. malariae), which are transmitted by the bite of an infective female Anopheles sp. mosquito. Most malaria infections in the United States occur in persons who have traveled to areas with ongoing transmission. Occasionally, cases occur in the United States through exposure to infected blood products, by congenital transmission, or by local mosquitoborne transmission. Malaria surveillance is conducted to identify episodes of local transmission and to guide prevention recommendations for travelers. Reporting Period Covered: Cases with onset of illness during 1997. Description of System: Malaria cases confirmed by blood smears are reported to local and/or state health departments by healthcare providers and/or laboratory staff. Case investigations are conducted by local and/or state health departments, and reports are transmitted to CDC through the National Malaria Surveillance System (NMSS). Data from NMSS serve as the basis for this report. Results: CDC received reports of 1,544 cases of malaria with onset of symptoms during 1997 among persons in the United States or one of its territories. This number represents an increase of 10.9% from the 1,392 cases reported for 1996. P. vivax, P. falciparum, P. malariae, and P. ovale were identified in 48.9%, 36.7%, 3.1%, and 2.0% of cases, respectively. More than one species was present in nine patients (0.6% of total). The infecting species was not determined in 134 (8.7%) cases. The number of reported malaria cases acquired in Africa increased by 38.0% (n=697) compared with 1996, and cases acquired in Asia increased by 12.9% (n=499) compared with 1996. Cases acquired in the Americas decreased by 9.3% (n=245) compared with 1996. Of 695 U.S. civilians who acquired malaria abroad, 144 (20.7%) reported that they had followed a chemoprophylactic drug regimen recommended by CDC for the area where they had traveled. Five patients became infected in the United States. Three of these cases were congenitally acquired; one was acquired by a blood transfusion; and one was acquired by a needle stick. Six deaths were attributed to malaria. Interpretation: The 10.9% increase in malaria cases in 1997 compared with 1996 resulted from increases in cases acquired in Africa and Asia. This increase could have resulted from local changes in disease transmission, increased travel to these regions, improved reporting from state and local health departments, or a decreased use of effective antimalarial chemoprophylaxis. In most reported cases, U.S. civilians who acquired infection abroad were not on an appropriate chemoprophylaxis regimen for the country where they acquired malaria. Public Health Actions: Additional information was obtained concerning the six fatal cases and the five infections acquired in the United States. Persons traveling to a malarious area should take the recommended chemoprophylaxis regimen and use personal protection measures to prevent mosquito bites. Any person who has been to a malarious area and who subsequently develops a fever or influenzalike symptoms should seek medical care immediately; investigation should include a blood smear for malaria. Malaria infections can be fatal if not diagnosed and treated promptly. Recommendations concerning prevention and treatment of malaria can be obtained from CDC. INTRODUCTIONMalaria is caused by infection with one or more of four species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, and P. malariae) that infect humans. The infection is transmitted by the bite of an infective female Anopheles sp. mosquito. Malaria infection remains a devastating global problem, with an estimated 300--500 million cases occurring annually. Forty-one percent of the world's population lives in areas where malaria is transmitted (i.e., parts of Africa, Asia, Central America and South America, Hispaniola, the Middle East, and Oceania), and approximately 1.5--2.7 million persons die of malaria each year (1). In previous years, malaria was endemic throughout much of the continental United States; an estimated 600,000 cases occurred during 1914 (2). During the late 1940s, a combination of improved socioeconomic conditions, water management, vectorcontrol efforts, and case management was successful at interrupting malaria transmission in the United States. Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate the reintroduction of transmission and to monitor patterns of antimalarial drug resistance seen among U.S. travelers. Through 1997, most cases of malaria diagnosed in the United States have been imported from regions of the world where malaria transmission is known to occur. Each year, several congenital infections and infections resulting from exposure to blood or blood products are reported in the United States. In addition, a few cases are reported that might have been acquired through local mosquitoborne transmission (3). State and/or local health departments and CDC thoroughly investigate all malaria cases acquired in the United States, and CDC conducts an analysis of all imported cases to detect trends in acquisition. This information has been used to guide malaria prevention recommendations for travelers abroad. For example, an increase in P. falciparum malaria among U.S. travelers to Africa, an area with increasing chloroquineresistance, prompted CDC to change the recommended chemoprophylaxis from chloroquine to mefloquine in 1990 (4). The signs and symptoms of malaria illness are variable, but most patients have fever. Other common symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. The diagnosis of malaria should be considered for any person who has any of these symptoms and has traveled to an area with known malaria transmission. Malaria should also be considered in the differential diagnosis when persons have a fever of unknown origin, regardless of their travel history. Untreated P. falciparum infections can rapidly progress to coma, renal failure, pulmonary edema, and death. Asymptomatic parasitemia can occur among persons who have been longterm residents of malarious areas. This report summarizes malaria cases reported to CDC with onset of symptoms in 1997. METHODSSources of Data Data regarding malaria cases are reported to both the National Malaria Surveillance System (NMSS) and the National Notifiable Diseases Surveillance System (NNDSS) (5). Although both systems rely on passive reporting, the numbers of reported cases might differ because of differences in the collection and transmission of data. A major difference in the data collected in these two systems is that NMSS receives more detailed clinical and epidemiologic data regarding each case (e.g., information concerning the area where the infected person has traveled). Cases of blood-smear--confirmed malaria are identified by healthcare providers and/or laboratories. Each slide-confirmed case is reported to local and/or state health departments and to CDC on a uniform case report form that contains clinical, laboratory, and epidemiologic information. CDC staff review all report forms at the time of receipt and request additional information if necessary (e.g., when no recent travel to a malarious country is reported). Reports of other cases are telephoned directly by health-care providers to CDC, usually when assistance with diagnosis or treatment is requested. All cases that have been acquired in the United States are investigated, including all induced and congenital cases and possible introduced or cryptic cases. Information derived from uniform case report forms is entered into a database and analyzed annually. Definition of Terms The following definitions are used in this report:
This report also uses terminology derived from the recommendations of the World Health Organization (6). Definitions of the following terms are included for reference.
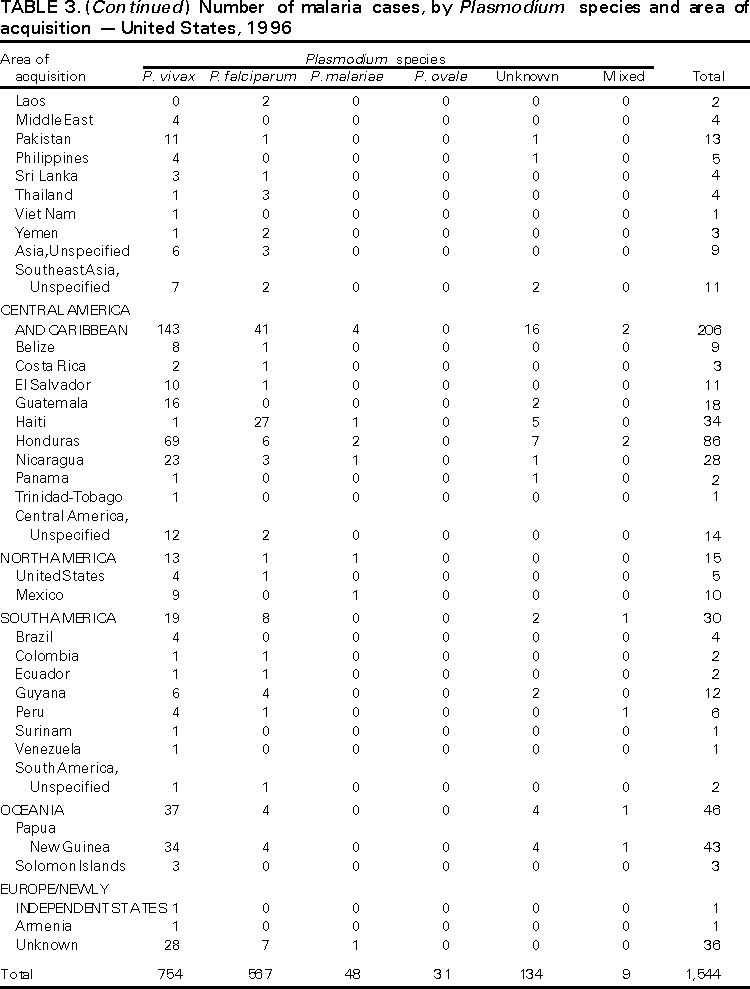
Microscopic Diagnosis of Malaria The early diagnosis of malaria requires that physicians consider malaria in the differential diagnosis of every patient who has fever; the evaluation of such a patient should include taking a comprehensive travel history. If malaria is suspected, a Giemsa-stained smear of the patient's peripheral blood should be examined for parasites. Thick and thin blood smears must be prepared properly because the accuracy of diagnosis depends on the quality of the blood film and the experience of the laboratory personnel.* (See Appendix A for proper procedures necessary for accurately diagnosing malaria). RESULTSGeneral Surveillance During 1997, CDC received reports of 1,544 malaria cases that had onset of symptoms among persons in the United States and its territories, representing a 10.9% increase from the 1,392 cases reported for 1996 (7). This incidence is the largest number of reported cases since 1980. In 1997, a total of 698 cases occurred in U.S. civilians compared with 618 cases reported for 1996. This incidence represents the largest number of U.S. civilian cases reported since 1968 (Table 1). The number of cases in foreign civilians decreased from 636 in 1996 to 592 in 1997 (Figure 1). Cases among U.S. military personnel also decreased from 32 in 1996 to 28 in 1997. In 226 cases, civilian or military status was not reported. Plasmodium Species The infecting species of Plasmodium was identified in 1,410 (91.3%) of the cases reported in 1997. P. vivax and P. falciparum were identified in blood smears from 48.9% and 36.7% of infected persons, respectively (Table 2). The 755 P. vivax cases reported for 1997 represented a 14.4% increase from the 660 cases in 1996, whereas the number of P. falciparum infections increased by 8.8% (from 521 in 1996 to 567 in 1997). Among 1,410 cases in which both the region of acquisition and the infecting species were known, 76.6% of infections acquired in Africa were attributed to P. falciparum; 12.4% were attributed to P. vivax. The converse was true of infections acquired in Asia and the Americas: 89.6% and 69.7% were attributed to P. vivax, and only 8.1% and 19.9% were attributed to P. falciparum, respectively. Region of Acquisition and of Diagnosis Approximately 98% (n=1,522) of all cases were classified as imported. Of 1,507 imported cases in which the region of acquisition was known, most 46.2% (n=697) were acquired in Africa, whereas 33.1% (n=499) and 16.2% (n=245) were acquired in Asia and the Americas, respectively (Table 3). The largest concentration of cases acquired in Africa (n=457; 65.6%) came from countries in West Africa, whereas most (n=410; 82.2%) of the cases acquired in Asia came from the Indian subcontinent. The other regions where imported cases of malaria were acquired were Central America and Caribbean (n=206; 13.7%), South America (n=30; 2.0%), and Oceania (n=46; 3.0%). Information regarding region of acquisition was missing for 32 (2.1%) of the imported cases. The number of reported malaria cases acquired in Africa increased by 19.1% (n=697) compared with 1996, and cases acquired in Asia increased by 12.9% (n=499) compared with 1996. Cases from the Americas decreased by 6.0% (n=251) in 1997 compared with 1996. In the United States, the six areas reporting the largest number of malaria cases were California (n=374), New York City (n=303), Florida (n=120), New York (n=84), Virginia (n=68), and Illinois (n=68) (Figure 2). Of these areas, only New York reported a decrease in cases in 1997 compared with 1996. This overall increase in reported number of cases might be a result of an increased rate of international travel, improved access to health care, or more sensitive surveillance. Interval Between Arrival and Illness Of those persons who became ill with malaria after arriving in the United States, both a) the interval between the date of arrival in the United States and onset of illness and b) the identification of the infecting Plasmodium species were known for 1,091 (71.7%) of the imported cases of malaria (Table 4). Symptoms began after arrival in the United States for 1,022 (93.7%) of these cases. Clinical malaria developed within 1 month after arrival in 344 (77.6%) of the 443 P. falciparum cases and in 124 (21.3%) of the 582 P. vivax cases (Table 4). Only 27 (2.6%) of the 1,022 persons became ill >1 year after returning to the United States. An additional 69 (6.3%) persons reported becoming ill before arriving in the United States. Imported Malaria Cases Imported Malaria in Military Personnel Twenty-eight cases of imported malaria in U.S. military personnel were reported for 1997. Of the 26 case-patients for whom information on use of chemoprophylaxis was available, 10 were not using any prophylaxis. Imported Malaria in Civilians A total of 1,287 imported malaria cases were reported among civilians. Of these, 695 (54.0%) occurred in U.S. residents, and 592 (46.0%) occurred in residents of other countries (Table 5). Of the 695 imported malaria cases in U.S. civilians, 353 (50.8%) had been acquired in Africa, an increase of 78.4% from cases reported in 1996. The Central American and Caribbean region accounted for 109 (15.7%) cases of imported malaria in U.S. civilians, whereas travel to Asia accounted for an additional 148 (21.3%) cases. Of the 592 imported cases among foreign civilians, most cases were acquired in either Africa (n=209; 35.3%) or Asia (n=295; 49.8%). Use of Antimalarial Chemoprophylaxis Use of Chemoprophylaxis Among U.S. Civilians. Information concerning use of chemoprophylaxis and area of travel was known for 620 (89.2%) of the 695 U.S. civilians who had imported malaria. Of these 620 persons, 356 (57.4%) had not taken any chemoprophylaxis, and 82 (13.2%) had not taken a drug recommended by CDC for the area visited. Only 144 (23.2%) U.S. civilians had taken a medication recommended by CDC (8). Data for the actual drug taken were missing for the remaining 38 (6.1%) travelers. Of the civilian patients on prophylaxis recommended by CDC, 118 had taken mefloquine weekly; nine had taken doxycycline daily; and 17 who had traveled only in areas where chloroquine-resistant malaria has not been documented had taken chloroquine weekly. Of the 82 patients taking a nonrecommended drug, information on the type of chemoprophylaxis used was known for 59. Of these 59 persons, 56 (94.9%) reported taking chloroquine during travel to an area where chloroquine resistance had been documented. Malaria Infection After Use of Recommended Prophylaxis. After taking a recommended antimalarial drug, 182 patients (144 U.S. civilians, 10 persons in the U.S. military, 10 foreign civilians, and 18 persons whose information regarding their status was missing) developed malaria. The infecting species was undetermined for 29 cases. Cases of P. vivax or P. ovale. Of the 182 patients who developed malaria after taking recommended chemoprophylaxis, 98 cases (53.8%) were caused by P. vivax (n=94) or P. ovale (n=4). Malaria case surveillance reports indicated that 16 (16.3%) of these patients were noncompliant with antimalarial prophylaxis. Fifty (51.0%) cases of P. vivax or P. ovale occurred >45 days after the case-patients arrived in the United States. These cases were consistent with relapsing infections and, thus, do not necessarily indicate prophylaxis failures. Because of insufficient information regarding 37 cases, it could not be determined whether these cases were relapsing infections. Eleven cases (11.2%) caused by P. vivax occurred within 45 days after the patient returned to the United States. Of these case-patients, seven were noncompliant with the recommended antimalarial chemoprophylaxis. The region of acquisition varied for the four case-patients reported to have complied (two from Central America, one from South America, one from Papua New Guinea). Serum drug levels could not be checked for any of these patients because blood samples were not available. The most likely explanations for these prophylaxis failures are either inappropriate dosing or unreported noncompliance. No evidence indicates the emergence of a new area of chloroquine-resistant P. vivax. The remaining 84 cases of malaria reported among persons who had taken a recommended antimalarial drug for chemoprophylaxis include 43 cases of P. falciparum, 8 cases of P. malariae, 4 cases of mixed infection, and 29 cases in which the infecting species was not identified. Cases of P. falciparum. Thirty-six of the 43 P. falciparum cases were acquired in Africa, two in Asia, two in Central America and Carribean, and three in South America. In 22 (51.2%) of the case-patients, noncompliance with antimalarials was reported; one of the case-patients died as a result of noncompliance. Twenty-one (48.8%) persons who reported taking the recommended prophylaxis regimen acquired P. falciparum infections; however, serum drug levels were not available for these patients. The most likely explanations for these failures are either inappropriate dosing or noncompliance. Purpose of Travel. The purpose of travel to malarious areas was reported for 469 (67.5%) of the 695 U.S. civilians with imported malaria (Table 6). Of the cases in U.S. civilians, the largest percentage (22.6%) of case-patients traveled to visit friends or relatives in malarious areas; the second and third largest percentages, 11.5% and 10.9%, traveled to tour and to do missionary work, respectively. Malaria Acquired in the United States Congenital Malaria Three cases of congenital malaria were reported in 1997. Case 1. In May 1997, a male neonate of 30 weeks' gestational age was delivered in New York City. Two days after delivery, he developed hyperbilirubinemia; fever was first noted two days after delivery, but no cause was identified. Fourteen days after delivery, his hemoglobin level had dropped to 9.0 g/dL with a platelet count of 65,000/mm3. The neonate received a blood transfusion; he was discharged and sent home. On May 20, 1997, the neonate was readmitted to the hospital. A malaria smear revealed P. vivax parasites. He was treated with chloroquine and recovered uneventfully. The neonate's mother, a native of India, was aged 38 years. She had returned from visiting India approximately 1 month before the birth of her son. During that trip, she was not taking antimalarial prophylaxis. A malaria smear of her peripheral blood was positive for P. vivax parasites. The mother responded well to chloroquine and primaquine therapy. Case 2. After an uneventful prenatal course, a full-term male infant was born by normal spontaneous vaginal delivery in June 1997 in Oakland, California. The infant was admitted to the hospital with fever 3 weeks after birth. He was reported as feeding well. On examination, he was noted to have mild icterus and occasional petechiae and purpura. His platelet count was 37,000/mm3. A peripheral blood smear revealed intraerythrocytic parasites consistent with P. vivax. The infant was treated with a 3-day course of chloroquine and recovered without complications. The mother was born in the Punjab Province of India. She had immigrated to the United States in July 1996 and had not traveled outside of California since. She did not report any malaria symptoms during her pregnancy; however, she recalled having episodes of febrile illness while she was in India. The most recent febrile episode had been 3 years before delivery. The diagnosis of P. vivax was confirmed by CDC. The mother's thick and thin blood smears were negative, and immunofluorescent assay titers were 1:64 to P. vivax, <1:16 to P. falciparum, <1:16 to P. malariae, and <1:16 to P. ovale. The mother was treated with a course of chloroquine followed by 14 daily doses of primaquine. Case 3. In September 1997, a male infant was born in New York City, the son of a native of India who had immigrated to New York 14 months before she delivered. The mother had no episodes of febrile illness after arriving in the United States. The infant developed fever 45 days after birth, and P. vivax malaria was diagnosed. He was successfully treated with chloroquine. The mother's peripheral blood smear was examined; however, no parasites were found. Serum samples from both the infant and mother tested positive by immunofluorescent assay. Cryptic Malaria No cases of cryptic malaria were reported in 1997. Induced Malaria Two cases of induced malaria were reported in 1997, one acquired by a needle stick and one by blood transfusion. Case 1. On February 24, 1997, a Filipino nurse, aged 56 years, was working in a hospital in California and sustained a needle-stick injury while starting an intravenous line. The patient was being treated for a P. vivax infection acquired in Honduras. The nurse had immigrated to the United States in 1970, and her last visit to the Philippines was more than 4 years before her illness. Although she had traveled internationally to other areas in the previous 4 years, she had not been to any malarious areas. The nurse developed fever and chills on March 10, and a blood smear demonstrated P. vivax parasites. She was successfully treated with a 3-day course of chloroquine and a 14-day course of primaquine. Case 2. In Missouri, a male resident of an extended care facility, aged 85 years, was hospitalized for acute gastrointestinal hemorrhage in October 1997. While he was hospitalized, he received five units of packed red blood cells and was then discharged. The patient was readmitted on November 1, 1997, with another episode of acute gastrointestinal hemorrhage and fever to 104 F (40.0 C). Peripheral blood smears were positive for intraerythrocytic parasites identified as P. falciparum rings and trophozoites. He was treated with oral quinine and doxycycline; this regimen was changed to intravenous quinidine gluconate and doxycycline the next day when his mental status deteriorated. A computed tomography scan indicated evidence of a cerebral vascular accident. He died on November 18, 1997. The patient had been a World War II veteran but had no recent history of international travel. All five units of packed red blood cells that he received were obtained from the same community blood center; four of the units were donated by U.S. military personnel and one by a long-time civilian donor. Sera from the donors were tested by immunofluorescent assay; one donor tested positive for P. falciparum >16,384. Deaths Attributed to Malaria Six deaths attributed to malaria were reported in 1997. Case 1. On February 21, 1997, a male in North Carolina, aged 20 years, reported to an infirmary approximately 2 weeks after returning from a 2-month holiday trip to his native Zimbabwe. The man was in Zimbabwe for approximately 2 months; he reported having taken mefloquine prophylaxis sporadically during the trip. Upon reporting to the infirmary, he had fever, chills, cough, and headache. He was referred to an urgent care center; an upper respiratory infection was diagnosed. The patient was sent home with a prescription for amoxicillin. Within 1 week, he returned to the infirmary with fevers to 103 F (39.4 C), chills, vomiting, diarrhea, and jaundice. He was referred to the hospital emergency department for evaluation; his platelet count was 10,000/mm3. The patient was started on broad spectrum antibiotics. Arrangements were made to transfer him to the university hospital; however, the transfer was delayed for 24 hours because no vacant bed was available at the hospital. A peripheral blood smear revealed intraerythrocytic parasites, which were thought to be either P. falciparum or Babesia microti. A decision to delay treatment for possible malaria was made, pending a reevaluation at the university hospital. Upon arriving at the referral hospital, the patient was alert. However, approximately 1 hour after arriving, his neurologic status began to deteriorate; he subsequently experienced respiratory and cardiac arrest. He was resuscitated, placed on a respirator, and transferred to the coronary care unit. Repeat blood smears were positive for P. falciparum with a parasitemia of 23%. Exchange blood transfusions were started that evening as well as an intravenous therapy of quinidine gluconate and doxycycline. The patient's neurologic status did not improve, and he died in early March. Case 2. On June 12, 1997, a man aged 57 years went to a hospital in Ohio 12 days after leaving The Gambia where he had spent 2 months performing missionary work. He reported having taken a prophylactic medication, but neither the name of the drug nor the regimen were known. On admission, his hemoglobin level was 11 g/dL; total bilirubin, 5.0 g/dL; and platelet count, 13,000/mm3. Peripheral blood smears revealed that 25% of the erythrocytes were parasitized with P. falciparum. The patient was started on intravenous quinidine gluconate and doxycycline and was being prepared for exchange transfusion when he experienced severe bradycardia followed by cardiac arrest. The initial resuscitation was successful; however, he then suffered a second cardiac arrest, and resuscitation was not successful. Case 3. In June 1997, a man from Wisconsin, aged 59 years, traveled to Kenya for a safari. He was given chloroquine for prophylaxis. On June 15, he became ill with gastroenteritis. On June 23, 3 days after leaving Kenya, he became ill again and sought medical care the following day. He was started on antibiotic treatment, pending results of blood and stool cultures. The antibiotic was changed to ciprofloxacin on June 27 because his symptoms had continued. The patient was admitted to a hospital in Wisconsin on June 28. Blood smears taken on admission revealed P. falciparum, and he was started on quinine sulfate, doxycycline, and clindamycin. The patient developed oliguria and metabolic acidosis 1 day after admission. He died of multiple organ failure on June 30. Case 4. On September 6, 1997, a man who is a native of Nigeria, went to a New York City emergency department with altered mental status. The man, aged 58 years, was known to have insulin-dependent diabetes. He had recently returned from Nigeria. Information regarding whether he had used chemoprophylaxis was not known. Four days before admission, the patient had gone to his primary physician because he was not feeling well. An upper respiratory infection was diagnosed, and he was started on doxycycline. On the day before admission, he began having diarrhea; his mental status also began deteriorating. On physical examination, the patient was unconscious, had a temperature of 98.2 F (36.8 C), blood pressure of 120/90, and a pulse of 90 beats per minute. No significant findings were revealed from the remainder of the physical examination. Laboratory tests indicated a hemoglobin level of 11.3 g/dL; white blood cell count, 6,300/mm3; platelet count, 49,000/mm3; total bilirubin, 3.4 g/dL; glucose, 444 mg/dL; blood urea nitrogen, 112 mg/dL; creatinine, 2.8 mg/dL; and potassium, 5.6 meq/L. Malaria blood smear was positive for P. falciparum parasites. The patient was started on intravenous normal saline, an insulin drip, and intravenous cefuroxime. He was subsequently intubated and placed on mechanical ventilation. Intravenous quinidine gluconate was ordered after obtaining the results of the malaria smear; however, before it could be started, the patient suffered a cardiac arrest in the emergency department. Resuscitation attempts were not successful. Cause of death was listed as diabetic ketoacidosis and malaria. Case 5. See Case 2 of the "Induced Malaria" section. Case 6. On December 14, 1997, an infant, aged 9 months, began to have fever and lethargy and was taken to a hospital in Florida. Two days before the symptoms began, he had immigrated from Nigeria. He had been recently treated for malaria with an unknown injectable medicine while he was in Nigeria. On examination, the infant had pallor, hepatosplenomegaly, lethargy, and a temperature of 104 F (40.0 C). Laboratory tests revealed a hemoglobin level of 5.3 g/dL and a white blood cell count of 63,000/mm3. Malaria blood smears initially tested positive for P. vivax; however, CDC was consulted by telephone and suggested that the infection was P. falciparum. The infant was intubated, started on intravenous quinidine gluconate, and administered a blood transfusion. A computed tomography scan indicated cerebral hemorrhage and herniation. The infant died on December 17. DISCUSSIONA total of 1,544 cases of malaria was reported to CDC for 1997, representing a 10.9% increase from the 1,392 cases reported for 1996. This change primarily resulted from an increase in cases acquired in Africa and Asia that might have resulted from improved reporting, increased international travel, changing patterns of travel (i.e., immigration from malarious areas or "adventure tourism"), or a decreased use of effective antimalarial chemoprophylaxis. Since 1992, the number of malaria case-patients who were not classified as U.S. military, U.S. civilian, or foreign civilian have been increasing (Table 1). The Malaria Case Surveillance Report was changed in 1996, possibly contributing to the increase from 1996 to 1997; however, this change does not account for the earlier increases. Unknown cases were classified as such because data were missing from the surveillance forms. One reason for conducting malaria surveillance is to monitor the emergence of drug resistance and the consequent failure of chemoprophylaxis; however, approximately 70% of imported malaria in U.S. civilians occurred in persons who were either not taking or taking nonrecommended prophylaxis for the region where they were traveling. Of the 144 persons who reported taking adequate prophylaxis, for 24 cases (i.e. 7 P. vivax, 13 P. falciparum, and 4 P. malariae), sufficient information was not available to determine whether they represented problems with compliance while using proper antimalarial chemoprophylaxis, reporting errors, or emerging drug resistance. However, no conclusive evidence existed to suggest a single national or regional source of infection among this group of patients. Health-care providers are encouraged to contact CDC at (770) 488-7788 whenever they suspect chemoprophylaxis failure, thus enabling measurement of serum drug levels of the antimalarial drug in question. The importance of taking proper precautions and chemoprophylaxis is underscored by five of the six fatal cases of malaria in the United States in 1997. An earlier review of deaths attributed to malaria in the United States identified several risk factors for fatal malaria, including failure to take recommended antimalarial chemoprophylaxis, refusal of or delay in seeking medical care, and misdiagnosis (9). Signs and symptoms of malaria often are vague, but fever is generally present. Other typical symptoms include headache, chills, increased sweating, back pain, myalgia, diarrhea, nausea, vomiting, and cough. Prompt diagnosis requires that malaria be included in the differential diagnosis of illness in a febrile person with a recent history of travel to a malarious area. Clinicians should ask febrile patients for a travel history, particularly when evaluating febrile illnesses in international visitors, immigrants, refugees, migrant laborers, and international travelers. Treatment for malaria should be initiated immediately after the diagnosis has been confirmed by a positive blood smear. Treatment should be determined on the basis of the infecting Plasmodium species, the probable geographic origin of the parasite, the parasite density, and the patient's clinical status (10). Although nonfalciparum malaria rarely causes complications, persons with P. falciparum infection are at risk for developing severe, lifethreatening complications. Healthcare workers are encouraged to consult appropriate sources for malaria treatment recommendations or to contact CDC's National Center for Infectious Diseases, Division of Parasitic Diseases, Malaria Epidemiology Branch (Box). AcknowledgmentThe authors acknowledge the state health departments, health-care providers, and public health laboratories for reporting this information to CDC. References
* To obtain confirmation diagnosis of blood smears from questionable cases and to obtain appropriate treatment recommendations, contact either your state or local health department or CDC's National Center for Infectious Diseases, Division of Parasitic Diseases, Malaria Epidemiology Branch; telephone (770) 488-7788. APPENDIXMicroscopic Procedures for Diagnosing Malaria To establish the diagnosis of malaria, a blood smear must be prepared from fresh blood obtained by pricking the finger (Figures A-1 and A-2).* The thin smear is fixed in methanol before staining; the thick smear is stained unfixed. Many hospitals have a Wright-Giemsa stain available, which is acceptable; however, Wright stain alone will not reliably stain Plasmodium parasites. For best results, the smear should be stained with a 3% Giemsa solution (pH of 7.2) for 30--45 minutes. In P. falciparum infections, the parasite density should be estimated by counting the percentage of red blood cells infected --- not the number of parasites --- under an oil immersion lens on a thin film. Thick blood smears are more sensitive in detecting malaria parasites because the blood is concentrated, allowing a greater volume of blood to be examined. However, thick smears are more difficult to read, and thin smears might be preferred by laboratories that have limited experience. Plasmodium parasites are always intracellular, and they demonstrate, if stained correctly, blue cytoplasm with a red chromatin dot. Common errors in reading malaria smears are caused by platelets overlying a red blood cell, concern about missing a positive slide, and misreading artifacts as parasites. Persons suspected of having malaria but whose blood smears do not demonstrate the presence of parasites should have blood smears repeated approximately every 12--24 hours for 3 consecutive days. If smears remain negative, then the diagnosis of malaria is unlikely. For rapid diagnosis, make the thick and thin smears on separate slides. Air dry the thin film, fix it with methyl alcohol, and immediately stain it. If no parasites are found on the thin film, wait until the thick film is dry and examine it for organisms that might not have been detected on the thin preparation. * In Figures A-1 and A-2, the hands are shown ungloved to better illustrate their placement during the procedures. However, wearing gloves while processing blood specimens is recommended to prevent transmission of bloodborne pathogens (MMWR 1988;37:377--82, 387--8 and MMWR 1987;36[No. S2]). Table 1 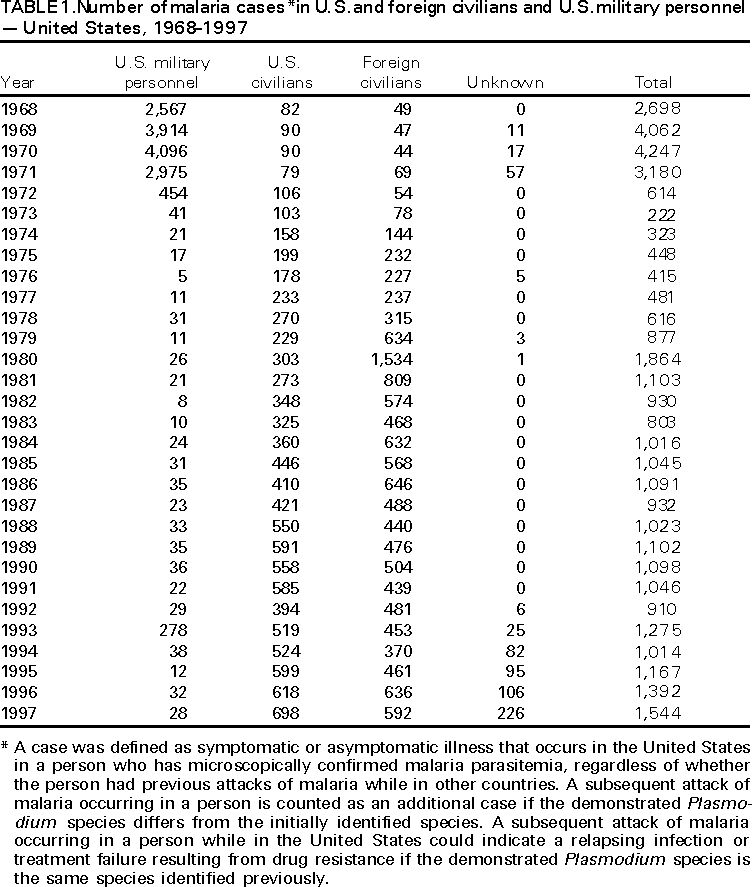 Return to top. Figure 1 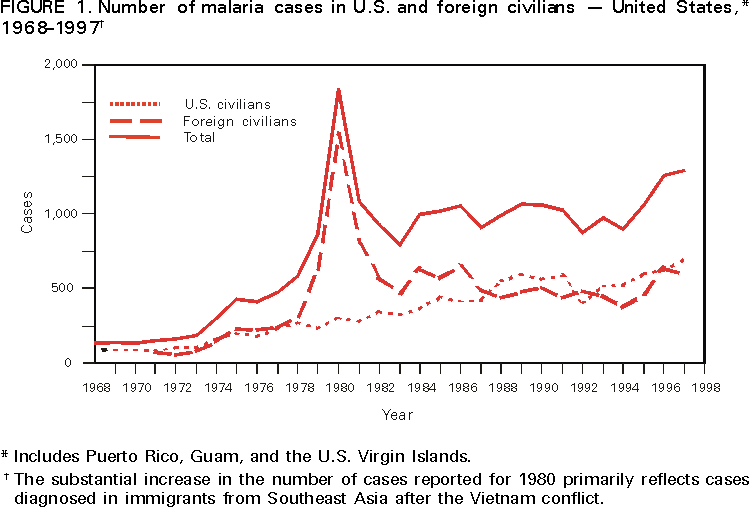 Return to top. Table 2 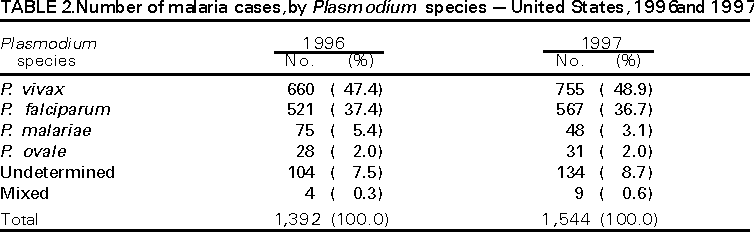 Return to top. Figure 2 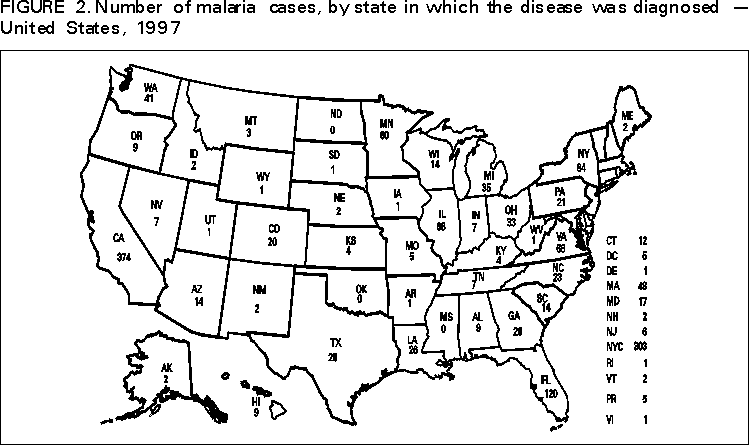 Return to top. Table 3 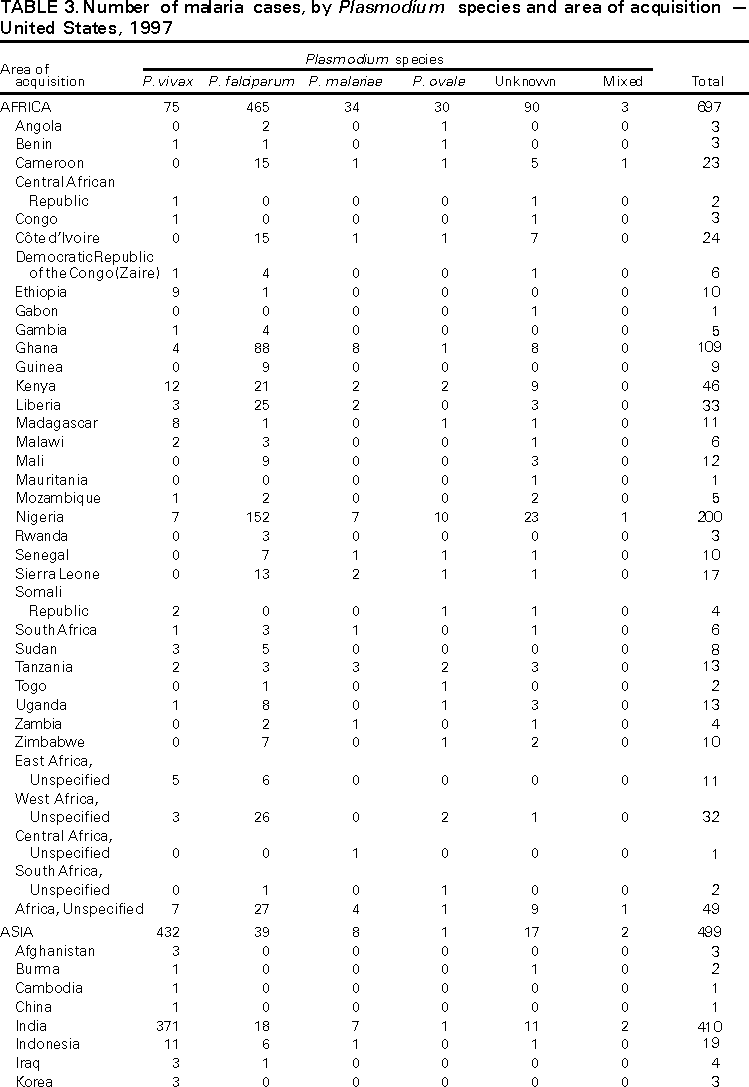
 Return to top. Table 4 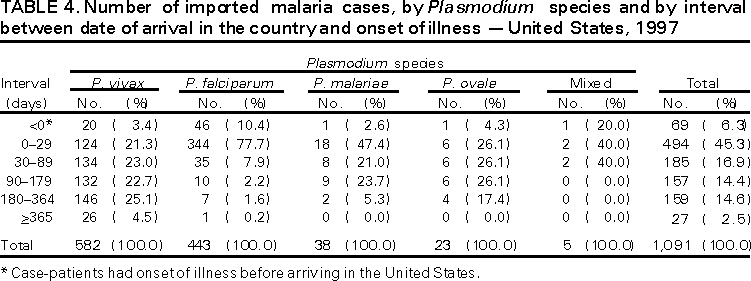 Return to top. Table 5 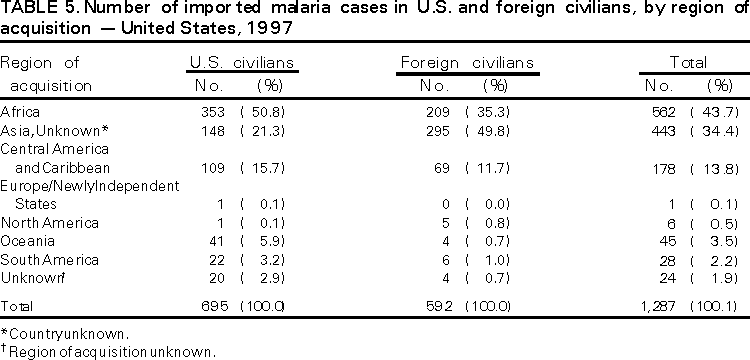 Return to top. Table 6 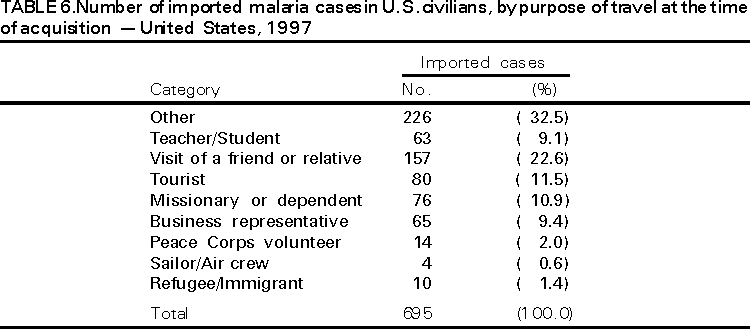 Return to top. Figure A-1 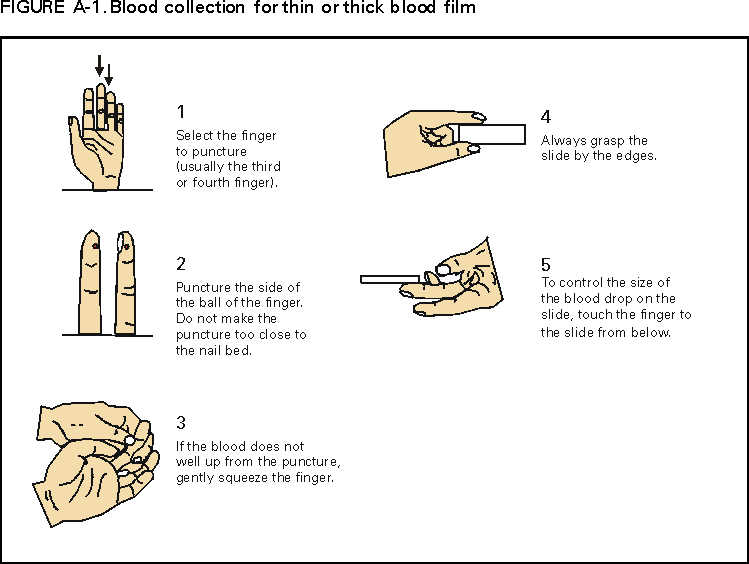 Return to top. Figure A-2 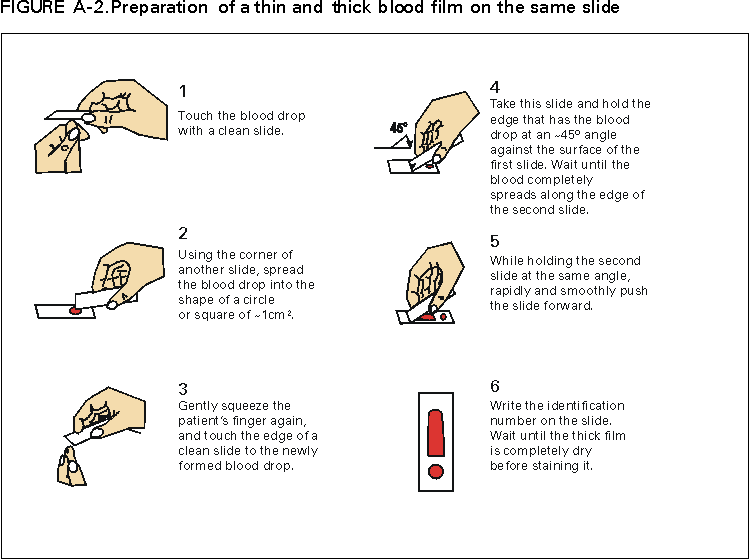 Return to top.
Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/21/2001 |
|||||||||
This page last reviewed 5/2/01
|