Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Update on Vaccine-Derived Polioviruses — Worldwide, April 2011–June 2012
In 1988, the World Health Assembly resolved to eradicate poliomyelitis worldwide (1). One of the main tools used in polio eradication efforts has been the live, attenuated oral poliovirus vaccine (OPV). This inexpensive vaccine is administered easily by mouth, makes recent recipients resistant to infection by wild polioviruses (WPVs), and provides long-term protection against paralytic disease through durable humoral immunity. Nonetheless, rare cases of vaccine-associated paralytic poliomyelitis can occur both among immunologically normal OPV recipients and their contacts and among persons who are immunodeficient. In addition, vaccine-derived polioviruses (VDPVs) can emerge to cause polio outbreaks in areas with low OPV coverage and can replicate for years in persons who are immunodeficient. This report updates previous surveillance summaries (2,3) and describes VDPVs detected worldwide during April 2011–June 2012. In 2011, a new outbreak of circulating VDPVs (cVDPVs) was identified in Yemen; a second VDPV isolate, related to a previously reported VDPV isolate (2), signaled an outbreak in Mozambique; and VDPV circulation reemerged in Madagascar. An outbreak that began in Somalia in 2008 continued until December 2011. Outbreaks in Nigeria and the Democratic Republic of the Congo (DRC) identified in 2005 and 2008, respectively, continued in 2012. Niger experienced a new cVDPV importation from Nigeria in 2011. Twelve newly identified persons in six middle-income countries were found to excrete immunodeficiency-associated VDPVs (iVDPVs), and VDPVs were found among healthy persons and environmental samples in 13 countries. To prevent VDPV emergence and spread, all countries should maintain high vaccination coverage against all three poliovirus serotypes; OPV use will be discontinued worldwide once all WPV transmission is interrupted (4).
Properties of VDPVs
VDPVs can cause paralytic polio in humans and have the potential for sustained circulation. VDPVs resemble WPVs biologically (2) and differ from most vaccine-related poliovirus (VRPV) isolates by having genetic properties consistent with prolonged replication or transmission. VDPVs were first identified by sequence analyses of poliovirus isolates. Because poliovirus genomes evolve at a rate of approximately 1% per year, VRPVs that differ from the corresponding OPV strain by >1% of nucleotide positions (determined by sequencing the genomic region that encodes the major viral surface protein [VP1]) are presumed to have replicated for at least 1 year in one or more persons after administration of an OPV dose and are VDPVs. One year is substantially longer than the normal period of vaccine virus replication of 4–6 weeks in an OPV recipient.
Three poliovirus serotypes have been identified: types 1, 2, and 3 (PV1, PV2, and PV3). Poliovirus isolates are grouped into three categories, based on the extent of divergence compared with the corresponding OPV strain: 1) VRPVs (<1% divergent [PV1 and PV3] or <0.6% divergent [PV2]); 2) VDPVs (VRPVs >1% divergent [PV1 and PV3] or >0.6% divergent [PV2]); and 3) WPVs (WPV1, WPV2, and WPV3, no genetic evidence of derivation from any vaccine strain) (2). VDPVs are further categorized as 1) cVDPVs, when evidence of person-to-person transmission in the community exists; 2) iVDPVs, which are isolated from persons with primary (B-cell) immunodeficiencies (defects in antibody production) who have prolonged VDPV infections; and 3) ambiguous VDPVs (aVDPVs), which are either clinical isolates from persons with no known immunodeficiency or sewage isolates whose source is unknown (2).
Virologic Testing for VDPVs
All poliovirus isolates are characterized by laboratories of the Global Polio Laboratory Network (GPLN) (5). The original protocol to screen for VDPVs, using a combination of molecular and antigenic methods, has largely been replaced by a real-time reverse transcription–polymerase chain reaction (rRT-PCR) nucleic acid amplification targeted to nucleotide substitutions that occur early in VDPV emergence (2). The original rRT-PCR procedure specifically amplified sequences typical of OPV strains; newer methods amplify sequences of potential VDPVs by targeting sequences that typically revert during replication of OPV in the human intestine. The rRT-PCR methods have been transferred to 60 of 146 GPLN laboratories (5). Candidate VDPVs following rRT-PCR screening are sequenced routinely in the VP1 region; the complete genome is sequenced if required for higher-resolution analysis.
cVDPVs
The number of countries with indigenous cVDPV circulation remained unchanged at six since the July 2009–June 2011 reporting period (2). Outbreaks in Afghanistan, Ethiopia, and India appeared to have stopped; outbreaks in DRC and Somalia continued; a large outbreak in Nigeria abated; new outbreaks were detected in Mozambique and Yemen, and genetic evidence indicated renewed VDPV circulation in Madagascar. Apart from a cVDPV exported from Nigeria into neighboring Niger, no cVDPVs were exported from the countries of emergence. In all but Mozambique, the emerging cVDPVs were PV2 (Figure).
DRC. Circulation of cVDPV2 in DRC continued in 2012, with a total of 64 cases detected since 2008. Since April 2011, 28 cVDPV2 isolates (0.7%–3.5% divergent) from persons with acute flaccid paralysis (AFP) have been detected, all in Katanga Province, where reported OPV coverage was low (6). An additional aVDPV2 isolate (0.7% divergent) from an AFP patient was detected in Katanga. As in Nigeria during 2005–2011, multiple independent VDPV2 emergences were identified in DRC.
Madagascar. Serial and concurrent emergences of VDPVs were first reported in Madagascar during 2002 (7). VDPV surveillance has been enhanced by virologic testing of stools collected from healthy children in southern Madagascar. Two recent genetically related VDPV isolates provide evidence of circulation.
Mozambique. A second VDPV isolate (4.3% divergent) related to a previously reported aVDPV isolate (2) was identified in a child with AFP, providing evidence of circulation during this reporting period.
Niger. One cVDPV2 (5.2% divergent) was isolated from a patient in southern Niger, near the border with Nigeria, with onset of AFP in November 2011. The isolate was closely related to cVDPVs circulating in nearby Kano and Jigawa states, Nigeria. As with the five previous cVDPV2 importations from Nigeria detected since May 2006 (2), no secondary cases were found in Niger.
Nigeria. Since 2005, a total of 378 AFP cases associated with a cVDPV2 outbreak (0.7%–6.5% divergent) were reported in 11 northern and three north-central states of Nigeria where routine vaccination with trivalent OPV (tOPV) coverage was low (<60%) (2). The outbreak peaked (153 cases) in 2009; 27 cases were detected in 2010, and 35 cases were detected in 2011. Only two cases had been detected as of June 2012, but 30 additional genetically distinct cVDPV2 isolates were obtained from environmental samples in the northern states of Kano and Sokoto. The outbreak is associated with approximately 25 independent VDPV2 emergences, at least seven of which led to cVDPV2 transmission (2).
Somalia. VDPV2 has been detected in Somalia since 2005. During April–December 2011, cVDPV2 (1.0%–3.5% divergent) were isolated from three patients with AFP and 14 contacts in the regions surrounding Mogadishu; all were derived from a single emergence.
Yemen. During April–October 2011 cVDPV2 (0.6%–1.6% divergent) were isolated from nine patients with AFP and one contact. The outbreak was derived from at least two independent emergences.
iVDPVs
Since the introduction of OPV in 1961, approximately 65 persons with primary immunodeficiencies have been found worldwide to be excreting iVDPVs (indicating prolonged infections); the majority of these immunodeficiencies were detected only after onset of AFP. After implementation of intensified surveillance for VDPVs and special studies of iVDPV excretion among persons with primary immunodeficiencies in developing and middle-income countries, detection of iVDPV infections increased from two during January 2008–June 2009 (3), to nine during July 2009–June 2011 (2), and to 12 during April 2011–June 2012.
China. A girl aged 11 months with common variable immunodeficiency developed AFP in February 2012, following 3 OPV doses. The patient was coinfected with iVDPV2 and iVDPV3.
Egypt. Surveillance for VDPVs in Egypt was enhanced during the reporting period by screening of persons with primary immunodeficiencies. An iVDPV1 was isolated from a boy aged 18 months with agammaglobulinemia after onset of AFP in May 2011, an iVDPV3 was isolated from a child aged 21 months with primary immunodeficiency after onset of AFP in April 2011, and a girl aged 3 months with agammaglobulinemia was found to be infected with iVDPV2 in 2011 but did not develop AFP. All three patients died.
India. A girl aged 6 months with primary immunodeficiency in West Bengal was infected with iVDPV2.
Iran. Iran has maintained sensitive clinical and laboratory surveillance to screen persons with primary immunodeficiencies for poliovirus infections. During April 2011–June 2012, four AFP patients were found to be excreting iVDPVs. A boy aged 6 years with primary immunodeficiency infected with an iVDPV2 developed AFP in May 2011. A boy aged 15 months with primary immunodeficiency and infected with an iVDPV2 developed AFP in June 2011, a boy aged 25 months with primary immunodeficiency who was coinfected with an iVDPV1 and iVDPV2 developed AFP in December 2011, and a boy aged 6 months with primary immunodeficiency and infected with an iVDPV2 developed AFP in March 2012. The last two boys died.
South Africa. A boy aged 10 months with agammaglobulinemia and infected with an iVDPV3 developed AFP in September 2011 and subsequently died.
Sri Lanka. A girl aged 8 years with common variable immunodeficiency and infected with an iVDPV3 developed AFP in 2011; she remained alive through mid-2012, with a last VDPV-positive specimen collected in March 2012.
West Bank and Gaza. A boy aged 1 year with severe combined immunodeficiency who had not developed AFP was found to be infected with an iVDPV2 in 2011. The patient died of immunodeficiency complications in January 2012.
aVDPVs
During April 2011–June 2012, aVDPVs were isolated in 12 countries (Table). The most divergent aVDPVs were continuations of lineages previously detected in sewage samples in Finland and Israel, two countries with high (>90%) polio vaccination coverage. The persons infected with the corresponding aVDPVs have not been identified. Detection of aVDPVs in settings (including local pockets) with low (<60%) polio vaccination coverage might signal cVDPV emergence and potential gaps in surveillance. Some aVDPVs, especially those with limited divergence in areas with high vaccination coverage and in patients with no known immunodeficiency, might represent limited spread of OPV virus or the upper limit of OPV sequence divergence in a single normal vaccine recipient or contact.
Argentina. An aVDPV1 was isolated in May 2011 from a paralyzed girl aged 15 months who had received 2 doses of OPV. Final diagnosis was botulism, and the child was no longer excreting VDPV.
DRC. An aVDPV1 (0.7% divergent) was isolated from an AFP patient with no known immunodeficiency in December 2011.
Finland. A highly divergent aVDPV2 (15.4%) was isolated from sewage samples collected in July 2011. This aVDPV2 isolate was related to aVDPV2 isolates detected during 2008−2011 in sewage samples, and nearly equivalent in divergence to aVDPV1 isolates and aVDPV3 isolates likely derived from a single tOPV dose (2).
India. In 2011, genetically distinct aVDPV2s (0.7%–1.1% divergent) were isolated from five AFP patients with no known immunodeficiency, and an aVDPV3 (1.5% divergent) was isolated from another AFP patient. In addition, both aVDPV1 (1.2% divergent) and aVDPV2 (0.7%–1.1% divergent) were isolated from sewage samples.
Israel. Two genetically distinct groups of highly divergent aVDPV2s had been detected in sewage samples in 1998 (group 1, 15.6%–16.2% divergent) and 2006 (group 2, 10.7%–11.2% divergent) (2,8). Group 1 virus was detected in sewage samples in September 2011. Group 2 virus has been not been detected in sewage samples since March 2011.
Madagascar. An aVDPV2 (0.7% divergent) genetically distinct from the cVDPV2s described in Madagascar was isolated from an unvaccinated healthy child in southern Madagascar.
Nigeria. Two aVDPV2s (0.7%–1.1% divergent) were isolated in November 2011 and May 2012 from two unvaccinated AFP patients in Niger and Edo states, providing evidence of continued cVDPV2 emergence. Two aVDPV2s unrelated to the known cVDPV2s were detected in Sokoto sewage samples in May and June 2012.
Peru. An aVDPV2 (2.2% divergent) was isolated from an AFP patient with no known immunodeficiency in April 2011.
South Sudan. An aVDPV2 (1.1% divergent) was isolated from an AFP patient in February 2012.
Sudan. An aVDPV2 (0.7% divergent) was isolated from an AFP patient in April 2012.
Yemen. Two genetically distinct aVDPV2s (1.0%–1.1% divergent) were isolated from AFP patients in separate communities in Yemen in September 2011 and February 2012, providing evidence of new cVDPV2 emergences. An aVDPV3 (2.3% divergent) was isolated from an AFP patient in April 2012.
Reported by
Polio Eradication Dept, World Health Organization, Geneva, Switzerland. Global Polio Laboratory Network. Div of Viral Diseases, National Center for Immunization and Respiratory Diseases; Global Immunization Div, Center for Global Health, CDC. Corresponding contributor: Olen M. Kew, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC, okew@cdc.gov, 404-639-3940.
Editorial Note
The World Health Organization convened a meeting in May 2012 to review the current understanding of VDPV emergence and transmission. Several key points that were reaffirmed are as follows: 1) the clinical signs and severity of paralysis associated with VDPV and WPV infections are indistinguishable; 2) cVDPVs pose the same public health threat as WPVs and require the same control measures; 3) persons with prolonged iVDPV infections can transmit poliovirus to others, raising the risk for VDPV circulation in settings of low population immunity to the corresponding poliovirus serotype; 4) existing surveillance suggests that prolonged iVDPV excretion is uncommon among persons with primary immunodeficiencies exposed to OPV; 5) the prevalence of long-term iVDPV excretors might be higher than detected by existing surveillance of persons with primary immunodeficiencies, as suggested by the detection of aVDPVs that closely resemble iVDPVs in sewage in Finland, Israel, and other countries with high poliovirus vaccine coverage; and 6) the development of treatment for prolonged iVDPV infections might facilitate detection of and access to those with infections.
Detection of genetically related VDPVs from different persons who are not close contacts, even if none of the infected persons had AFP, is evidence of VDPV circulation and should prompt the same vaccination response as detection of VDPVs in AFP patients or WPV in persons or the environment. Key risk factors for cVDPV emergence and spread are 1) development of immunity gaps arising from low poliovirus vaccine coverage; 2) prior elimination of the corresponding WPV serotype, which also eliminates immunity from natural infection; 3) emphasis on use in supplementary immunization activities (SIAs)* of monovalent OPV (mOPV) and bivalent OPV (bOPV) types 1 and 3 vaccine formulations, which can lead to immunity gaps to PV2 (2,9); and 4) insensitive AFP surveillance. Many of these factors exist in areas of insecurity, such as in parts of Somalia and Yemen. In this context, VDPV2s present the greatest threat for emergence (2), and it was emphasized at the meeting that routine immunization should be strengthened and, for the immediate future, regular SIAs using tOPV (which efficiently closes population immunity gaps when used at high coverage rates) should be conducted.
Since 1999, when the last WPV2 case was identified, all cases of poliomyelitis involving PV2 have been associated with the use of tOPV primarily in the context of low poliovirus vaccine coverage. To prevent emergence and transmission of VDPV2 as progress is made toward WPV eradication, the Strategic Advisory Group of Experts advising the World Health Organization has recommended simultaneous global cessation of tOPV use in both routine vaccination services and SIAs and switching to bOPV as soon as it is safe to do so (10). Prerequisites for a switch from tOPV to bOPV include 1) strong evidence of cessation of all cVDPV2 transmission based on certification-standard global poliovirus surveillance, including environmental surveillance, to detect VDPV and WPV infections as a supplement to AFP surveillance in appropriate settings; 2) maintenance of high poliovirus vaccination coverage against all three poliovirus serotypes in all countries; 3) increased use of inactivated poliovirus vaccine to maintain immunity to all three serotypes; and 4) strategic deployment of OPV stockpiles in the event of outbreaks.
References
- CDC. Progress toward interruption of wild poliovirus transmission—worldwide, January 2011–March 2012. MMWR 2012;61:353–7.
- CDC. Update on vaccine-derived polioviruses—worldwide, July 2009–June 2011. MMWR 2011;60:846–50.
- CDC. Update on vaccine-derived polioviruses—worldwide, January 2008–June 2009. MMWR 2009;58:1002–6.
- CDC. Update on vaccine-derived polioviruses. MMWR 2006;55:1093–7.
- CDC. Tracking progress toward global polio eradication, 2010–2011. MMWR 2012;61:265–9.
- CDC. Progress toward global polio eradication—Africa, 2011. MMWR 2012;61:190–4.
- Rakoto-Andrianarivelo M, Gumede N, Jegouic S, et al. Reemergence of recombinant vaccine-derived poliovirus outbreak in Madagascar. J Infect Dis 2008;197:1427–35.
- Shulman LM, Manor Y, Sofer D, et al. Neurovirulent vaccine-derived polioviruses in sewage from highly immune populations. PLoS One 2006;1:e69.
- Jenkins HE, Aylward RB, Gasasira MB, et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N Engl J Med 2010;362:2360–9.
- World Health Organization. Meeting of the Strategic Advisory Group of Experts, April 2012—conclusions and recommendations. Wkly Epidemiol Rec 2012;87:201–16.
* SIAs are mass vaccination campaigns conducted in a short period (days to weeks) during which a dose of OPV is administered to all children aged <5 years, regardless of previous vaccination history. Campaigns can be conducted nationally or in portions of a country.
What is already known on this topic?
Genetically divergent vaccine-derived polioviruses (VDPVs) are detected by poliovirus surveillance and have biological properties indistinguishable from wild polioviruses. High coverage with poliovirus vaccine can eradicate all WPVs and prevent circulating VDPV (cVDPV) outbreaks, but prolonged immunodeficiency-associated VDPV (iVDPV) infections will occur as long as live, attenuated oral poliovirus vaccine (OPV) is used.
What is added by this report?
During April 2011–June 2012, although cVDPV outbreaks in three countries appear to have stopped, and the large outbreak in Nigeria has been reduced sharply, outbreaks continued in the Democratic Republic of the Congo and Somalia, and new outbreaks were detected in three countries. Twelve new prolonged iVDPV infections were detected, with increasing numbers found in developing and middle-income countries. Approximately 85% of VDPVs have been type 2.
What are the implications for public health practice?
Circulation of VDPVs does not present an insurmountable obstacle to polio eradication but instead is a symptom of low poliovirus vaccine coverage. Circulating VDPV outbreaks can be prevented and controlled by high OPV coverage. By contrast, only cessation of OPV use will prevent prolonged iVDPV infections. The World Health Organization has responded to the global VDPV risk by 1) increasing the frequency of trivalent OPV (tOPV) supplemental immunization activities (SIAs) in countries with low routine tOPV coverage, 2) considering a shift to bivalent OPV (type 1 plus type 3) as soon as possible, 3) encouraging the ongoing shift to inactivated poliovirus vaccine, 4) enhancing VDPV surveillance, and 5) encouraging development of antiviral drugs to clear prolonged iVDPV infections.
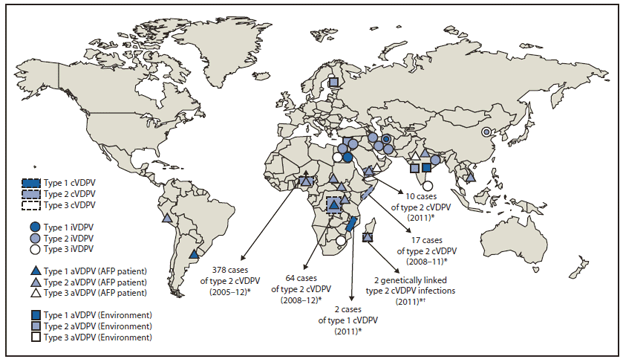
Abbreviations: cVDPV = circulating VDPV; iVDPV = immunodeficiency-associated VDPV; aVDPV = ambiguous VDPV; AFP = Acute flaccid paralysis.
* Spread of cVDPVs followed the elimination of the corresponding serotype of indigenous wild poliovirus, but with continued introduction of oral poliovirus vaccine into communities with growing immunity gaps. All of the cVDPV outbreaks were detected first by the laboratory, using sequence data and evolutionary analyses.
† Circulation was inferred because two isolates from healthy children shared a common VDPV2 ancestor but were genetically divergent.
Alternate Text: The figure above shows vaccine-derived polioviruses (VDPVs) detected worldwide, during April 2011-June 2012. The number of countries with indigenous circulating VDPVs (cVDPVs) remained unchanged at six since the July 2009-June 2011 reporting period. Outbreaks in Afghanistan, Ethiopia, and India appeared to have stopped; outbreaks in Democratic Republic of the Congo and Somalia continued; a large outbreak in Nigeria abated; new outbreaks were detected in Mozambique and Yemen, and genetic evidence indicated renewed VDPV circulation in Madagascar. Apart from a cVDPV exported from Nigeria into neighboring Niger, no cVDPVs were exported from the countries of emergence. In all but Mozambique, the emerging cVDPVs were poliovirus serotype 2.
|
TABLE. (Continued) Vaccine-derived polioviruses (VDPVs) detected worldwide, April 2011–June 2012 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Category |
Country |
Year(s) detected* |
Source (total cases or specimens)† |
Serotype |
No. of isolates§ April 2011–June 2012 |
VP1 divergence from Sabin OPV strain (%) |
Routine coverage with 3 doses of poliovirus vaccine (%)¶ |
Estimated duration of VDPV replication** |
Current status (date of last outbreak case, last patient isolate, or last environmental sample) |
||
|
Cases |
Contacts |
Non-AFP Source |
|||||||||
|
aVDPV |
Argentina |
2011 |
AFP patient |
1 |
1 |
— |
— |
1.1 |
95 |
<1 yr |
May 15, 2011 |
|
Burundi |
2011 |
AFP patient |
2 |
1 |
— |
— |
0.7 |
94 |
<1 yr |
December 15, 2011 |
|
|
DRC |
2011 |
AFP patient |
1 |
1 |
— |
— |
0.7 |
78 |
<1 yr |
December 20, 2011 |
|
|
Finland§§§§ |
2008–2011 |
Environment |
2 |
— |
— |
1 |
>11 |
99 (IPV) |
>15 yrs |
July 25, 2011 |
|
|
India |
2011 |
AFP patients |
2 |
4 |
— |
— |
0.7–1.1 |
70 |
0.5–1 yr |
November 25, 2011 |
|
|
2011 |
AFP patient |
3 |
1 |
— |
— |
1.3–1.5 |
1–1.5 yr |
October 7, 2011 |
|||
|
2011 |
Environment |
2 |
— |
— |
6 |
0.7–1.1 |
0.5–1 yr |
April 2011 |
|||
|
2011 |
Environment |
1 |
— |
— |
1 |
1.2 |
~1 yr |
April 2011 |
|||
|
2012 |
Environment |
2 |
— |
— |
4 |
0.7–1.1 |
0.5–1 yr |
May 2012 |
|||
|
Israel¶¶¶¶ |
1998–2011 |
Environment |
2 |
— |
— |
8 |
15.6–16.2 |
94 (IPV) |
<15 yrs |
September 20, 2011 |
|
|
Madagascar |
2011 |
Healthy child |
2 |
— |
— |
1 |
0.7 |
88 |
<1 yr |
May 20, 2011 |
|
|
Nigeria |
2011–2012 |
AFP patient |
2 |
2 |
— |
— |
0.7–1.1 |
73 |
0.5–1 yr |
May 22, 2012 |
|
|
Environment |
2 |
— |
— |
2 |
0.7–0.8 |
<1 yr |
May 28, 2012 |
||||
|
Peru |
2011 |
AFP patient |
2 |
1 |
— |
— |
2.2 |
91 |
2 yrs |
April 11, 2011 |
|
|
South Sudan |
2012 |
AFP patient |
2 |
1 |
— |
— |
1.1 |
46 |
~1 yr |
February 24, 2012 |
|
|
Sudan |
2012 |
AFP patient |
2 |
1 |
— |
— |
0.7 |
93 |
<1 yr |
April 1, 2012 |
|
|
Viet Nam |
2012 |
AFP patient |
2 |
1 |
— |
— |
0.7 |
96 |
<1 yr |
February 14, 2012 |
|
|
Yemen |
2011 |
AFP patients |
2 |
2 |
— |
— |
1.0–1.1 |
81 |
~1 yr |
February 2, 2012 |
|
|
2012 |
AFP patient |
3 |
1 |
— |
— |
2.3 |
2 yrs |
April 27, 2012 |
|||
|
Abbreviations: cVDPV = circulating VDPV; iVDPV = immunodeficiency-associated VDPV; aVDPV = ambiguous VDPV; OPV = oral poliovirus vaccine; IPV = inactivated poliovirus vaccine; AFP = acute flaccid paralysis; AGG = agammaglobulinemia; CVID = common variable immunodeficiency; DRC = Democratic Republic of the Congo; PID = primary immunodeficiency; SCID = severe combined immunodeficiency; VP1 = viral protein 1 (the major viral surface protein). * Total years detected and cumulative totals for previously reported cVDPV outbreaks (Democratic Republic of the Congo, Ethiopia, Nigeria). † Outbreaks list total cVDPV cases. Some VDPV case isolates from outbreak periods might be listed as aVDPVs. § Total cases for VDPV-positive specimens from AFP cases and total VDPV-positive samples for environmental (sewage) samples. ¶ Based on 2011 data from the World Health Organization (WHO) Vaccine Preventable Diseases Monitoring System (2012 global summary) and WHO-UNICEF coverage estimates, available at http://www.who.int/immunization_monitoring/en/globalsummary/countryprofileselect.cfm. National data might not reflect weaknesses at subnational levels. ** Duration of cVDPV circulation was estimated from the extent of VP1 nucleotide divergence from the corresponding Sabin OPV strain; duration of replication was estimated from the clinical record by assuming that exposure was from the initial receipt of OPV; duration of a VDPV replication was estimated from sequence data. †† All cVDPV isolates from DRC, Madagascar, Mozambique, Niger, Nigeria, Somalia, and Yemen were vaccine/nonvaccine recombinants. §§ Previously reported outbreak. Additional information available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6025a3.htm. ¶¶ Circulation was inferred because two isolates from healthy children shared a common VDPV2 ancestor but were genetically divergent. *** The first isolate was initially categorized as an aVDPV1. Additional information available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6025a3.htm?s_cid=mm6025a3_w. Isolation of a related isolate from a second patient confirmed VDPV1 circulation. ††† Importation from Nigeria. §§§ Previously reported outbreak. Additional information available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6025a3.htm. ¶¶¶ Count does not include 29 cases with <10 substitutions in VP1 detected before 2010. **** Previously reported outbreak. Additional information available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6025a3.htm. †††† None of the iVDPV isolates appeared to be vaccine/nonvaccine recombinants. §§§§ Previously reported. Additional information available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6025a3.htm. ¶¶¶¶ Previously reported. Additional information available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6025a3.htm. |
|||||||||||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


