 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Multistate Outbreak of Monkeypox --- Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003On July 2, 2003, this report was posted on the MMWR website (http://www.cdc.gov/mmwr). CDC and state and local health departments continue to investigate cases of monkeypox among persons who had contact with wild or exotic mammalian pets or persons with monkeypox (1--3). This report updates epidemiologic, laboratory, and smallpox vaccine use data for U.S. cases, and summarizes the laboratory-based evidence implicating imported African rodents as the probable source of the outbreak. As of July 2, a total of 81 cases of monkeypox have been reported to CDC from Wisconsin (39), Indiana (22), Illinois (16), Missouri (two), Kansas (one), and Ohio (one) (Figure); these include 32 (40%) cases laboratory-confirmed at CDC and 49 (60%) suspect and probable cases under investigation (Table). One case was excluded from those reported in the previous update because it met the exclusion criteria outlined in the updated case definition, and three were added (3). Of the 81 cases, 43 (53%) were among females; the median age was 27 years (range: 1--51 years). Age data were unavailable for one patient. Among 78 patients for whom data were available, 19 (24%) were hospitalized. The previously reported child with painful adenopathy associated with diffuse pox lesions improved clinically and was discharged from the hospital after 6 days (3). Confirmatory testing of skin rash lesions at CDC was positive for monkeypox virus. Of the 81 reported cases, 31 have been laboratory confirmed at CDC for monkeypox by detection of virus in skin rash lesions by using culture, polymerase chain reaction (PCR), immunohistochemical testing, and/or electron microscopy; one case was confirmed by virus isolation and PCR testing of an oropharyngeal specimen. The number of confirmed cases by state includes Wisconsin (14), Indiana (seven), Illinois (eight), Missouri (two), and Kansas (one). For these laboratory-confirmed cases, the onset of illness ranged from May 16 to June 20. All confirmed patients reported a rash, and all but one reported at least one other clinical sign or symptom, including fever, respiratory symptoms, and/or lymphadenopathy. The median incubation period* was 12 days (range: 1--31 days). The majority of patients with confirmed monkeypox reported exposure to wild or exotic mammals, including prairie dogs; some patients also had contact with other persons with monkeypox virus infection in a household setting. No cases of monkeypox that could be attributed exclusively to person-to-person contact have been confirmed. Use of Smallpox VaccineTo prevent further transmission of monkeypox, 28 residents of six states have received smallpox vaccine since June 13; recipients included 26 adults and two children. Vaccine was administered to two laboratory workers and two health-care workers pre-exposure and to 24 persons post-exposure (10 health-care workers, seven household contacts, three laboratory workers, two public health veterinarians, one public health worker, and one work contact). One child vaccinated post-exposure had a rash 6 days after vaccination; PCR testing of skin lesions from the child was positive for monkeypox virus. The child lived in a household with two ill prairie dogs and an adult with laboratory-confirmed monkeypox virus infection. One prairie dog had been present in the household for approximately 1 year and became ill after the introduction of a second ill prairie dog into the home. The child's period of exposure began 25 days before vaccination, when the ill prairie dog was brought into the home; the child's rash began 12 days after the onset of rash illness in the adult household member. Animal Laboratory TestingTraceback investigations have implicated a shipment of animals from Ghana that was imported to Texas on April 9 as the probable source of introduction of monkeypox virus into the United States (1,2). The shipment contained approximately 800 small mammals of nine different species, including six genera of African rodents. These rodent genera included rope squirrels (Funiscuirus sp.), tree squirrels (Heliosciurus sp.), Gambian giant rats (Cricetomys sp.), brushtail porcupines (Atherurus sp.), dormice (Graphiurus sp.), and striped mice (Hybomys sp.). Gambian rats from this shipment were kept in close proximity to prairie dogs at an Illinois animal vendor implicated in the sale of infected prairie dogs. CDC laboratory testing of some animals by using PCR and virus isolation demonstrated that one Gambian giant rat, three dormice, and two rope squirrels from the April 9 importation were infected with monkeypox virus. Evaluation of other animals associated with the shipment is ongoing. Evidence of infection was found in some animals that had been separated from the rest of the shipment on the day of their arrival into the United States, indicating early and possibly widespread infection among the remaining animals in the shipment. The laboratory investigation confirmed that multiple animal species are susceptible to infection with monkeypox virus. CDC had recommended previously that state health officials place quarantines on commercial facilities or households that had infected animals or received African rodents from the April 9 shipment (1). CDC has issued guidance on the quarantine and euthanasia of all rodents from the April 9 shipment, as well as prairie dogs that were exposed to the imported rodents or other animals with illnesses consistent with the case definition for monkeypox (http://www.cdc.gov/ncidod/monkeypox/quarantineremoval.htm). Animals that are euthanized according to the guidance should be incinerated and not buried in a landfill or backyard setting. CDC and the Food and Drug Administration issued a joint order on June 11 prohibiting the importation of any African rodent. In addition, the order prohibits the sale and transport within the United States of prairie dogs and six genera of African rodents (http://www.cdc.gov/ncidod/monkeypox/pdf/embargo.pdf ). To prevent the spread of monkeypox virus into domestic or wild animal populations, the order also prohibits releasing any of these animals into the wild. State and local health departments or departments of agriculture should be consulted for guidance on the safe disposal of animals. The joint order remains in effect regardless of the actions related to the guidance for quarantine and euthanasia of animals of concern. Health-care providers, veterinarians, and public-health officials who suspect monkeypox in animals or humans should report such cases to their state and local health departments. State health departments should report suspect cases to CDC, telephone 770-488-7100. An updated case definition with revised case exclusion criteria is available at http://www.cdc.gov/ncidod/monkeypox/index.htm. Clinical specimens should be submitted for testing after consultation with the state and local health department. Interpretation of laboratory results requires completion of specimen submission forms, which are available at http://www.cdc.gov/ncidod/monkeypox/diagspecimens.htm. Reported by: State and local health departments. Monkeypox investigation team, CDC. References
* Defined as first possible exposure date to illness onset date; however, some persons reported intermittent or continuous exposure. Table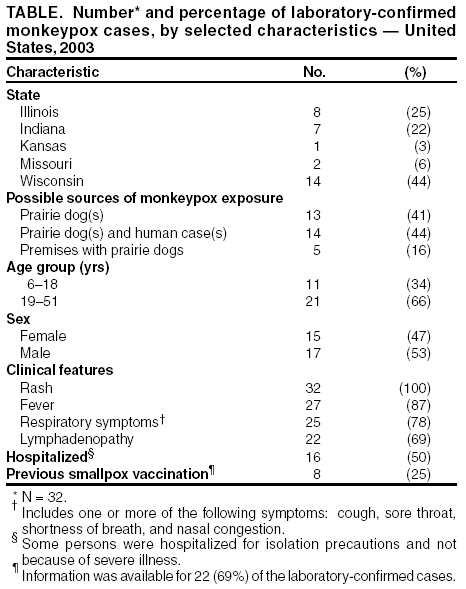 Return to top. Figure 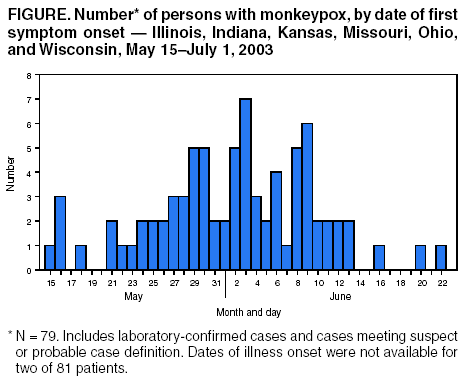 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/3/2003 |
|||||||||
This page last reviewed 7/3/2003
|