 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevention of Plague: Recommendations of the Advisory Committee on Immunization Practices (ACIP)The following CDC staff members prepared this report: Kenneth L. Gage, Ph.D. David T. Dennis, M.D., M.P.H. Theodore F. Tsai, M.D., M.P.H. Division of Vector-Borne Infectious Diseases National Center for Infectious Diseases Summary These revised recommendations by the Advisory Committee on Immunization Practices concerning prevention of plague update previous recommendations (MMWR 1982;31:301-4). This report includes information and recommendations on vaccination, public health practices, and medical treatment to prevent plague among humans. INTRODUCTION Plague is an acute, often fatal, and potentially epidemic disease caused by infection with Yersinia pestis. Plague exists in natural enzootic cycles involving wild rodents and their fleas in certain regions of Asia, Africa, the Americas, and extreme southeastern Europe near the Caspian Sea (Figure_1). These natural cycles may be inapparent, with no transmission to humans, or associated with sporadic transmission to humans. Epidemics of plague occasionally occur when the disease spreads from wild rodents into populations of rats (genus Rattus) that live near human habitation. Because of the high case-fatality rate and the epidemic potential of this disease, plague is designated a Class I notifiable disease and thus is subject to International Health Regulations. These regulations require that all suspected cases be reported to and investigated by public health authorities and that confirmed cases be reported to the World Health Organization (WHO) in Geneva, Switzerland. In the United States, suspected cases of plague should be reported to state health departments, which in turn notify CDC. All cases subsequently confirmed by laboratory analysis are reported by CDC to WHO. CHARACTERISTICS OF PLAGUE Etiologic Agent Y. pestis is a gram-negative coccobacillus belonging to the Enterobacteriaceae. This bacterium has several chromosomal and plasmid-associated factors that are essential to its virulence and survival in mammalian hosts and flea vectors (1,2). Modes of Transmission and Description of Disease The most common mode of transmission of Y. pestis to humans is by the bite of infectious fleas. Less frequently, infection is caused by a) direct contact with infectious body fluids or tissues while handling an infected animal or b) inhaling infectious respiratory droplets or other infectious materials (e.g., laboratory-generated aerosols containing Y. pestis). Infection causes a severe febrile illness characterized by headache, myalgia, malaise, shaking chills, prostration, and gastrointestinal symptoms. The three principal clinical presentations of plague are bubonic, septicemic, and pneumonic. Bubonic plague, characterized by development of an acute regional lymphadenopathy, or bubo, is the most common clinical form of disease, accounting for 80%-90% of cases in the United States. Buboes typically involve lymph nodes that drain the site of initial infection and are most often located in the inguinal, axillary, or cervical regions (3,4). The incubation period for bubonic plague usually ranges from and rarely exceeds 2 to 6 days. The case-fatality rate for infected persons who are not treated is 50%-60% (3). Septicemic plague, which occurs when Y. pestis invades and continues to multiply in the bloodstream, can occur secondarily to bubonic plague or can develop without detectable lymphadenopathy (i.e., primary septicemic plague) (3-5). In the United States during 1947-1977, approximately 10% of plague patients presented with septicemic plague; approximately 50% of these persons died as a result of disease (3). Complications of this form of plague include septic shock, consumptive coagulopathy, meningitis, and coma (3). Pneumonic plague is the least common but most dangerous and fatal form of the disease. It can develop as a secondary complication of septicemic plague or result from inhalation of infectious respiratory droplets expelled from a human or animal that has plague pneumonia. Signs of pneumonic plague include severe pneumonia accompanied by high fever, dyspnea, and often hemoptysis. The incubation period for primary pneumonic plague is 1-3 days. Patients who do not receive adequate treatment within 18 hours after onset of respiratory symptoms are unlikely to survive (3). Ecology Y. pestis is maintained in complex enzootic and epizootic transmission cycles involving susceptible wild rodents and their fleas (3,6). Risk for plague in humans is greatest when epizootics cause high mortality in commensal rat populations, thereby forcing infected rat fleas (Xenopsylla cheopis) to seek alternative hosts, including humans. These episodes of high mortality among rats do not contribute to maintenance of Y. pestis in nature (3). Other animals, including carnivores and rabbits, occasionally become infected with Y. pestis but are only incidental hosts. However, carnivores may contribute to the transfer of infected fleas from one geographic area to another (7). Epidemiology During 1980-1994, a total of 18,739 cases of plague in humans were reported to WHO from 20 countries (average: 1,087 cases per year) (Table_1) (8). Plague is underreported in many countries, particularly those in which surveillance and laboratory capabilities are inadequate. Epidemics are most likely to occur in areas that have poor sanitary conditions and large populations of rats. Isolated cases in humans also can result from exposure to infected wild or domestic animals or their fleas. Outbreaks of plague have been reported recently from Africa, Asia, and South America (8). In the United States, 341 cases of plague in humans were reported to CDC during 1970-1995 (average: 13 cases per year) (Figure_2). Of these cases, 80% occurred in the southwestern states of New Mexico, Arizona, and Colorado (9,10; CDC, unpublished data). Another 9% of cases were reported from California. Nine other western states reported limited numbers of cases. Modes of transmission were determined for 284 of these case-patients and included flea bite * (n=222; 78%); direct contact with infected animals (n=56; 20%); and inhalation of infectious respiratory droplets or other airborne materials from infected animals (n=7; 2%). Five of the seven persons who were infected by inhalation were exposed to infected domestic cats, and a sixth person (a veterinarian) also may have had such an exposure. In the United States, most cases of plague in humans occur in the summer months, when risk for exposure to infected fleas is greatest. The majority of these cases, especially those in the Southwest, are acquired in or near the patient's residence (9,10). Risks for acquiring the disease are associated with conditions that provide food and shelter for plague-susceptible rodents near human dwellings (6,9-12). Less often, plague is acquired while working or while participating in recreational activities, the latter having occurred most often among patients from California (13). PLAGUE VACCINE History of Plague Vaccines Killed bacteria have been used in plague vaccines since 1896. However, only one vaccine -- a formalin-inactivated preparation -- is currently licensed for use in the United States. This vaccine (plague vaccine, USP) was manufactured by Cutter Biologicals (a division of Miles Laboratories, Inc.) but is now available only from Greer Laboratories, Inc. The killed or inactivated plague vaccine is prepared from Y. pestis organisms grown in artificial media and then inactivated in formaldehyde. The inactivated bacteria are suspended in a 0.9% sodium chloride solution at a concentration of 1.8 to 2.2 x 109 bacteria per mL of vaccine. The vaccine also contains trace amounts of media components (e.g., beef-heart infusion, soytone {or phytone}, casamino acids, sodium sulfite {Na subscript 2 SO subscript 3}, L-cysteine hydrochloride, and yeast extract) and 0.5% phenol as a preservative. Live Y. pestis vaccines composed of presumably avirulent strains also have been developed (14). However, none of these vaccines is commercially available, and their safety and efficacy have not been adequately tested. Immunogenicity and Efficacy The efficacy of the inactivated plague vaccine in humans has not been measured in controlled studies. Completion of such studies in the United States is unlikely because of the low incidence of plague in this country. A retrospective study of U.S. military personnel who served in Vietnam provides some indirect evidence for the efficacy of the inactivated vaccine (manufactured by Cutter Laboratories) in preventing flea-borne plague (15). During 1961-1971, only eight cases of plague were diagnosed among U.S. personnel in Vietnam who had received plague vaccine (i.e., one case per 106 person-years of exposure). U.S. military personnel in Vietnam were considered to be at risk for exposure to Y. pestis-infected fleas because
Researchers have not determined whether vaccination protects against infectious droplets or aerosols generated in laboratories or against exposure to infectious respiratory droplets from patients who have pneumonic plague. At least two persons vaccinated with a previous formulation of the inactivated vaccine contracted pneumonic plague following exposure to Y. pestis (17,18). Whether these cases developed after the patients inhaled infectious droplets or as complications of septicemic plague was not reported. Persons potentially exposed to patients who have pneumonic plague or to Y. pestis aerosols in the laboratory should be administered a 7-day course of antimicrobial therapy, regardless of vaccination history. Antimicrobials recommended for prophylaxis include tetracycline, doxycycline, or trimethoprim-sulfamethoxazole (19). Laboratory studies involving animals suggest that induction of antibodies to the fraction 1 capsular antigen (F1) of Y. pestis following vaccination is a strong indicator of protection (18,20-25). Data from one study indicated that 90% of rats that had anti-F1 titers greater than or equal to 128 by PHA survived injection with 1 x 103 to 5 x 105 virulent Y. pestis, compared with 46% of rats with titers of 32-64 and 6% of rats with titers of less than or equal to 16 (24). Data from other studies reveal that 11 of 14 Hanuman langurs and six of seven African green vervets that had anti-F1 titers greater than or equal to 128 survived similar challenges (18,24,26,27). Mice vaccinated with F1 antigen expressed by a recombinant Escherichia coli clone containing the F1 gene of Y. pestis also were protected against challenge by virulent Y. pestis (28). Although data from previous studies have correlated the induction of adequate anti-F1 antibody titers with protection against plague, recent data indicate that immune responses to other Y. pestis antigens (e.g., V antigen) also might provide protection in laboratory animals (29). The extent to which plague vaccine induces such immune responses, however, has not been adequately determined. Studies of passively immunized animals also suggest that an anti-F1 titer greater than or equal to 128 is protective (18). For these indirect potency tests, sera (0.5 mL) from immunized mice, guinea pigs, monkeys, or humans are injected into 10 test mice. The object of this indirect mouse potency test is to determine whether anti-F1 antibodies present in the sera of the immunized animals protects the recipient mice from a subcutaneous challenge with virulent Y. pestis (20,30,31). Results are expressed as a mouse protection index (MPI), which is calculated by dividing the percentage of mortality among the 10 test mice by the average day of death (20,30). The lower the index, the greater the level of protection; MPIs less than or equal to 10 are considered protective (18). Data concerning protective antibodies in humans are limited. Data from one study indicated that 15 of 23 persons vaccinated with two doses of inactivated plague vaccine (manufactured by Cutter Biologicals) developed antibodies that were protective for mice (i.e., MPI less than or equal to 10) (18). A second study examined serum antibody levels among 29 persons who were administered three injections of the same vaccine (1.0 mL for the initial vaccination and 0.2 mL for vaccination 90 and 270 days later); 90% of vaccinees had detectable PHA titers within 15 days after the second injection, and 93% had such titers within 4 days after the third injection (20). The titers for each of the 29 vaccinees were not reported, but serum samples from five vaccine recipients who had titers greater than or equal to 128 yielded MPI values less than or equal to 10. Serum samples from five subjects who had PHA titers less than 128 did not protect mice in potency tests (20). Data from another study of 117 military personnel who received multiple doses of the inactivated plague vaccine (manufactured by Cutter Biologicals) indicated that 84 (72%) developed PHA titers ranging from 1,024 to 16,382, a total of 23 (20%) had titers from 64 to 512, and nine (8%) did not develop a titer in response to vaccine (32). Each of these 117 persons had received an initial series of three injections, followed by repeated booster injections at about 6-month intervals (average: five booster doses per person). Serum samples from 16 of these persons were tested in indirect mouse potency tests. All six vaccinees with PHA titers less than 128 had MPI values greater than 10, and four of the 10 subjects with titers greater than or equal to 128 had MPIs less than or equal to 10. A recent study conducted among U.S. military personnel compared MPI values in serum samples from four groups: persons vaccinated with one of three different lots of the inactivated vaccine manufactured by Greer Laboratories and a fourth group of persons administered the vaccine formerly marketed by Cutter Biologicals (Table_2) (Greer Laboratories, Inc., unpublished data). Subjects were vaccinated with two doses approximately 30 days apart. Humoral immune responses were measured in serum samples obtained 4 weeks after the second dose. Indirect potency tests demonstrated protective levels of antibody (MPI values less than or equal to 10) in sera of only 55%-58% of vaccinees. MPI values did not differ significantly by vaccine lot or manufacturer. Vaccine Administration Indications Persons should be vaccinated only if they are at high risk for exposure. Thus, vaccine is recommended for persons in the following high-risk groups:
in this report because of uncertainties about the efficacy of the vaccine (see Alternatives to Vaccination and Supplemental Protection). If vaccinees are believed to have been exposed to plague or if their risk for exposure is exceptionally high, prophylactic antibiotic use should be considered as a supplement to vaccination. Vaccination is not intended as a method of controlling plague epidemics because several months are required to complete the primary vaccination series and to develop adequate levels of protective antibodies. Routine vaccination is not necessary for persons living in areas in which plague is enzootic (e.g., the western United States). Vaccination is not indicated for hospital staff or other medical personnel in such areas, nor for most travelers to countries in which cases of plague have been reported, ** particularly if their itineraries are limited to urban areas and modern hotel accommodations. Supply and Storage Plague vaccine is manufactured by Greer Laboratories, Inc., P.O. Box 800, Lenoir, N.C. 28645-0800. The vaccine is shipped refrigerated in 20-mL vials and should be stored at 35-46 F (2-8 C). The vaccine should not be frozen. Primary Vaccination All injections should be administered intramuscularly, preferably in a deltoid muscle. Primary vaccination consists of a series of three injections (Table_3). Recommended dosages are available only for persons 18-61 years of age; dosage recommendations for other age groups have not been formulated because data are insufficient. No published data are available concerning the interactions of plague vaccine with drugs or other biologics administered concurrently. The simultaneous administration of plague vaccine with other vaccines that are likely to be reactogenic should be avoided. Booster Doses and Monitoring Antibody Levels The proportion of vaccinees whose serum produces an MPI less than or equal to 10 in the indirect mouse potency test after three doses of vaccine is unknown. PHA antibody titers decrease soon after vaccination and could drop below the presumably protective level of 128 within a few months (20). Booster doses of 0.2 mL each can be administered three times at approximately 6-month intervals when vaccinees have continuing high risk for exposure, especially for those persons who have PHA titers of less than 128. Additional booster doses can be administered at 1- to 2-year intervals to persons who remain at risk for infection. Persons considering being vaccinated should be advised that they may have difficulty having their PHA titers evaluated because only a few public health and research laboratories routinely perform this test. Adverse Reactions Adverse reactions following injection of the first dose of plague vaccine generally are mild, but the frequency and severity of such events can increase with repeated doses (33). Common adverse reactions include local erythema and induration at the site of injection, malaise, headache, fever, and lymphadenopathy (14). These reactions usually do not persist for greater than 48 hours. Severe reactions to plague vaccine occur in a limited number of recipients, most often in persons who receive repeated doses (33). Data regarding adverse reactions have been obtained largely from a study conducted during 1950-1971 that examined 1,219 persons who received a total of 18,751 injections (including primary-series and booster doses) of one or more of three different formalin-inactivated vaccine preparations (manufactured by Cutter Biologicals) (33). These preparations differed primarily in the growth media, strains of Y. pestis, and methods of standardization used in their preparation. The most recent of these three vaccine preparations, used during 1967-1971, was produced using essentially the same methods as the current Greer Laboratories vaccine. Although some local reactions (i.e., edema, induration at the site of injection, or both) were observed in 29% of the study population, moderately severe to severe local reactions were rare (occurring after 0.15% of injections). Severe local reactions included various combinations of a) greater than 12-cm edema and/or induration; b) inflammatory involvement of elbow, forearm, and/or clavicular fossa; and c) inflammation causing greater than 50% limitation of use of the arm. Seven (0.04%) additional injections resulted in immediate reactions characterized by transient swelling and redness radiating from the injection site. Six of these seven reactions occurred in the same person. Twenty percent of vaccine recipients had some systemic reactions (e.g., malaise, mild headache, generalized myalgia and arthralgia, fever, chills, and nausea). Of the 18,751 individual injections administered during the study, 68 resulted in severe systemic reactions (0.4% of total injections) that were characterized by one or more of the following manifestations: severe headache, generalized myalgia and arthralgia, malaise, nausea and vomiting, diarrhea, shaking chills, and oral temperatures greater than or equal to 101 F (greater than or equal to 38 C). Fourteen instances of anaphylaxis (0.07% of total injections) also were reported and were characterized by urticaria, bronchospasm, and hypotension. Anaphylaxis occurred within 30 minutes of inoculation and was of short duration because patients received prompt medical treatment. Fifty-three persons received only the more recently manufactured vaccine preparation, which is similar to the current Greer Laboratories vaccine. Local reactions occurred in 3% of persons who received this vaccine; 1% of persons had systemic reactions. Information regarding the severity of the local and systematic reactions occurring in this group was not reported. Although a few reports of sterile abscesses occurring following administration of plague vaccine were received by the former manufacturer (Cutter Biologicals), no such abscesses were identified in this study (Greer Laboratories, Inc., plague vaccine package insert). Plague vaccine has not been reported to cause death; however, one person who had a history of idiopathic anaphylaxis died several hours after receiving a third dose of plague vaccine (34). This person also had received Japanese encephalitis vaccine 2 days before death. The cause of death was not identified at autopsy. The frequency and severity of adverse reactions among military personnel 18-61 years of age who were administered the current Greer Laboratories vaccine are comparable to those previously reported for the Cutter Biologicals vaccine (Greer Laboratories, Inc., unpublished data). Persons who were administered vaccine manufactured by Greer Laboratories were initially injected with a 1.0-mL dose and then were administered a second, 0.2-mL dose 30 days later (Table_4). Adverse reactions generally were mild and transient and occurred less frequently after the second 0.2-mL injection than after the initial 1.0-mL dose (Table_4). No data concerning adverse events are available for the third primary dose or for booster injections of the Greer Laboratories vaccine. Adverse reactions should be reported to the Vaccine Adverse Event Reporting System (VAERS). VAERS reporting forms and information can be obtained by calling (800) 822-7967. Precautions and Contraindications Precautions to prevent adverse reactions should include review of the vaccinee's history of hypersensitivity to plague vaccine and its components. Epinephrine should be available for immediate use in the event of anaphylaxis or other allergic reactions to the vaccine. The safety and immunogenicity of the vaccine for persons less than 18 years of age have not been evaluated. The effects of plague vaccine on the developing fetus also are unknown. Pregnant women who cannot avoid high-risk situations should be advised of risk-reduction practices and should be vaccinated only if the potential benefits of vaccination outweigh potential risks to the fetus. Persons who are immunocompromised or who are receiving immunosuppressive therapy may not develop protective levels of antibody following vaccination. Whenever possible, antibody levels determined by PHA should be obtained to determine whether additional doses beyond the primary series should be administered. Plague vaccine should not be administered to persons who have a history of hypersensitivity to the vaccine or its components. Persons who have an acute febrile illness (e.g., influenza) should not be vaccinated until they have recovered fully. ALTERNATIVES TO VACCINATION AND SUPPLEMENTAL PROTECTION Vaccination is usually unnecessary when appropriate preventive measures are taken. Vaccinated persons should follow the preventive measures discussed in the following sections because of uncertainties about the efficacy of plague vaccine. Residents of Areas in Which Plague Is Enzootic In the western United States, most persons with plague become infected when rodent plague epizootics occur near their residences. Recommended means of reducing the risk for acquiring plague in and around homes include
Persons Who Participate in Outdoor Activities Hikers, campers, and other persons who participate in outdoor recreational activities in areas where plague is enzootic should a) avoid handling sick or dead animals, b) avoid rodent nests and burrows, c) use insect repellents containing N, N-diethyl-m-toluamide (DEET) on skin and repellents or appropriate insecticidal sprays on clothing, and d) treat accompanying pets with appropriate insecticides. Hunters should always wear gloves when handling dead animals. Medical Personnel and Persons Having Close Contact with Infected Persons Persons suspected of having pneumonic plague should be maintained under respiratory droplet precautions for 48 hours after antibiotic treatment begins (35). Persons who have confirmed cases of pneumonic plague should be kept under droplet precautions until sputum cultures are negative (35,36). Patients in whom pneumonic plague has been excluded warrant standard precautions only (35). Prophylactic antibiotics should be administered to persons who have had close exposure (i.e., within 6.5 feet {2 meters}) to persons suspected of having pneumonic plague (Table_5) (19,36). Persons who have not had such exposure are unlikely to become infected but should be monitored closely. Laboratory Workers Routine bacteriologic work involving plague can be performed in biosafety level 2 laboratories. Standard precautions (e.g., the use of a biological safety cabinet to contain aerosols that are generated unintentionally) are sufficient to prevent clinical laboratory workers from being infected with Y. pestis. Few laboratory-associated cases have been reported; these cases have involved unusual exposures in clinical diagnostic laboratories or in laboratories that conduct research involving live Y. pestis (37). Persons Who Work with Potentially Infected Animals These workers should be informed on how to minimize their exposure to the tissues and fleas of potentially infective animals. Precautionary measures include a) avoiding areas where high mortality in commensal rat populations has been observed, b) wearing gloves when handling animals, c) applying insect repellents containing DEET to clothes and skin, and d) treating clothes with appropriate insecticides. Persons Who Work in Veterinary Clinics Persons working in veterinary practices in areas where plague is enzootic should be educated on the risks of handling cats infected with Y. pestis. Such persons should wear gloves and eye protection and take appropriate respiratory precautions when examining cats that have fever and obvious acute lymphadenopathy, oral lesions, or pneumonia (9). Persons Living, Working, or Traveling in Other Countries In most countries of Africa, Asia, and South America in which plague is enzootic, the risk for acquiring plague is greatest in semiarid grassland or mountainous areas. The likelihood of plague spreading from wild-rodent foci into village or urban centers is greatest when a) environmental conditions precipitate an increase in or movement of rodent populations, thus leading to increased population densities of plague-susceptible rodents and their fleas or b) natural disasters or other events interrupt routine sanitary practices. Whenever possible, persons living in regions where plague is enzootic should avoid rodent-infested locations, especially if unusually high numbers of dead rodents have been reported. Persons who must work in such areas should avoid handling sick and dead rodents and use repellents and appropriate insecticides to reduce their risk for being bitten by fleas. Travelers to plague-endemic areas generally are at low risk for infection with Y. pestis. Persons who travel to these areas should avoid rat-infested sites with recently reported cases of plague among humans, especially those sites at which dead rats have been observed. To reduce the likelihood of being bitten by fleas, travelers can apply insect repellents to skin and repellents and insecticides to clothing and outer bedding. Short-term prophylactic use of antibiotics should be considered only for circumstances of exceptionally high risk of exposure to plague. CONCLUSION Plague is an acute bacterial illness that is typically transmitted to humans by the bites of infectious fleas, direct contact with infected animals, or inhalation of infectious respiratory droplets. In most instances, plague can be prevented by using an appropriate combination of
Although various modifications of killed plague vaccine have been available since World War II, its efficacy in protecting humans has not been adequately demonstrated. Pneumonic plague has developed in at least two persons despite vaccination, indicating that the vaccine may not be effective in the prevention of infection via inhalation of infectious respiratory droplets or other airborne materials. However, indirect evidence indicates that plague vaccine is effective for preventing flea-borne transmission of disease. Thus, plague vaccine is recommended for laboratory personnel who routinely are exposed to viable Y. pestis. Vaccine also should be considered for persons who regularly have contact with the wild-rodent hosts of plague or their fleas in areas where plague is enzootic or epizootic. References
Persons who develop inguinal buboes or recall being bitten by fleas are considered to have been infected via flea bite. ** For a current listing of countries, consult the most recent issue of WHO's Weekly Epidemiological Record. Current information also is available from the Division of Quarantine, National Center for Infectious Diseases (NCID) (telephone: {404} 639-8100) or from the Division of Vector-Borne Infectious Diseases, NCID (telephone: {970} 221-6400). Figure_1 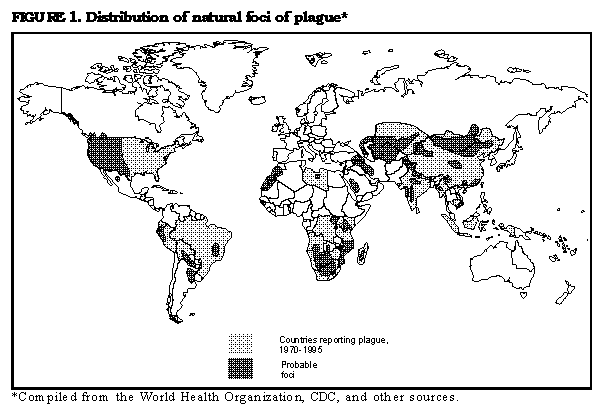 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Reported cases of plague in humans, by country -- 1980-1994 *
=======================================================================================
Continent Country No. of cases No. of deaths
-----------------------------------------------------------
Africa Angola 27 4
Botswana 173 12
Kenya 49 10
Libya 8 0
Madagascar 1,390 302
Malawi 9 0
Mozambique 216 3
South Africa 19 1
Tanzania 4,964 419
Uganda 660 48
Zaire 2,242 513
Zambia 1 1
Zimbabwe 397 1
Total 10,155 1,344
America Bolivia 189 27
Brazil 700 9
Ecuador 83 3
Peru 1,722 112
United States 229 33
Total 2,923 184
Asia China 252 76
India 876 54
Kazakhstan 10 4
Mongolia 59 19
Myanmar 1,160 14
Vietnam 3,304 158
Total 5,661 325
World Total 18,739 1,853
-----------------------------------------------------------
* World Health Organization. Human plague in 1994. Wkly Epidemiol Rec 1996;22:165-72.
=======================================================================================
Return to top. Figure_2 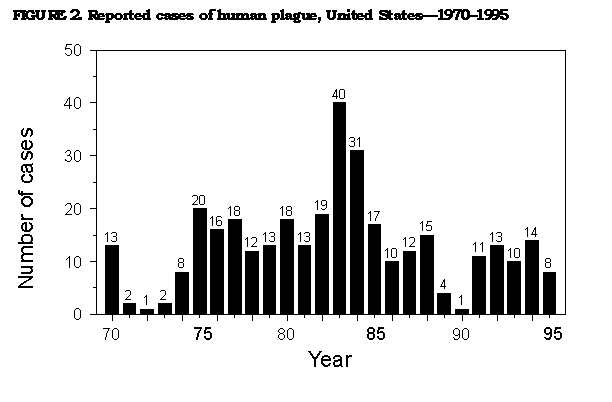 Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Mouse protection index (MPI) values for serum samples collected from
human vaccinees who were administered two doses of plague vaccine *+
===========================================================================================
Geometric Percentage of
Manufacturer Lot no. No. of subjects MPI MPI values protective MPIs &
--------------------------------------------------------------------------------------
Cutter 1 22 8.88 1.7-18.9 45
Greer 1 19 8.69 0.0-17.9 58
2 20 9.84 0.0-20.8 55
3 20 8.92 0.0-17.9 55
--------------------------------------------------------------------------------------
* Initial dose=1.0 mL; second dose=0.2 mL. The second dose was administered approximately
30 days after the first; sera were tested 57-58 days after the first injection.
+ Greer laboratories, Inc., unpublished data.
& MPIs <=10 are considered protective.
===========================================================================================
Return to top. Table_3 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size. TABLE 3. Recommended dosages of plague vaccine for primary vaccination of persons 18-61 * years of age ===================================================================================== Dose number Amount (mL) Recommended time of injection --------------------------------------------------------------- 1 1.0 NA + 2 0.2 1-3 months after first injection 3 0.2 5-6 months after second injection --------------------------------------------------------------- * Recommendations are not given for other age groups because data are insufficient. + Not applicable. ===================================================================================== Return to top. Table_4 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 4. Percentage of vaccine recipients reporting local and systemic reactions
occurring within 48 hours of vaccination with inactivated plague vaccine *+
======================================================================================
Percentage of recipients
-------------------------
Dose 1 & Dose 2 @
Reaction (N=67) (N=59)
---------------------------------------------------
Local
Tenderness 71.6 18.6
Decreased arm motion 11.9 1.7
Erythema 4.5 0.0
Warmth 3.0 1.7
Edema 1.5 0.0
Systemic
Headache 19.4 6.8
Nausea 13.4 3.4
Malaise 10.4 5.1
Dizziness 6.0 0.0
Chills 4.5 3.4
Joint ache 4.5 0.0
Muscle ache 4.5 0.0
Anorexia 1.5 0.0
Diarrhea 1.5 0.0
Vomiting 1.5 0.0
---------------------------------------------------
* Greer Laboratories, Inc.
+ Among adults 18-61 years of age.
& Vaccinees received 1.0 mL of vaccine.
@ Vaccinees received 0.2 mL of vaccine, which was administered 30 days after dose 1.
======================================================================================
Return to top. Table_5 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 5. Recommendations for prophylactic antimicrobial therapy for persons
exposed to Yersinia pestis,* by agent and age
===================================================================================================
Antimicrobial agent Age Dosage
-------------------------------------------------------------------------------------------------
Tetracycline + Adults & 2 g/day in two or four equal
doses at 12- or 6-hour
intervals for 7 days, by mouth
Children @ 25-50 mg/kg/day in two or
four equal doses at 12- or
6-hour intervals, respectively,
for 7 days, by mouth
Doxycycline Adults & 100-200 mg/day in two equal
doses at 12-hour intervals for
7 days, by mouth
Children @ 2-4 mg/kg/day in two equal
doses at 12-hour intervals for
7 days, by mouth
Trimethroprim- Adults & 1.6-3.2 g/day
sulfamethoxazole sulfamethoxazole component
in two equal doses at 12-hour
intervals for 7 days, by mouth
Children ** 40 mg/kg/day
sulfamethoxazole component
in two equal doses at 12-hour
intervals for 7 days, by mouth
-------------------------------------------------------------------------------------------------
* Information is for single exposures. Prolonged use of antimicrobial prophylaxis should be
supervised by a physician in consultation with public health officials.
+ Use of tetracycline during pregnancy should be avoided.
& Persons >= 18 years of age.
@ Persons 9-17 years of age.
** Persons >= 2 months to 18 years of age.
===================================================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|