 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Notice to Readers: Protocols for Confirmation of Reactive Rapid HIV TestsOn November 7, 2002, the Food and Drug Administration (FDA) announced approval of the OraQuick® Rapid HIV-1 Antibody Test (OraSure Technologies, Inc., Bethlehem, Pennsylvania) for use by trained personnel as a point-of-care test to aid in the diagnosis of infection with human immunodeficiency virus type 1 (HIV-1). Subsequently, two other rapid HIV tests have been approved by FDA: the Reveal™ HIV-1 Antibody Test (MedMira Laboratories, Halifax, Nova Scotia) and the Uni-Gold Recombigen™ HIV Test (Trinity Biotech, Wicklow, Ireland). All reactive rapid HIV test results require confirmatory testing. CDC described protocols for confirming reactive rapid HIV tests based on a consultation convened in January 2003 with expert laboratory scientists, FDA, and the Centers for Medicare and Medicaid Services (1). These protocols recommend 1) confirmation of all reactive rapid HIV test results with either Western blot (WB) or immunofluorescent assay (IFA), even if an enzyme immunoassay (EIA) screening test is negative, and 2) follow-up testing for persons with negative or indeterminate confirmatory test results, with a blood specimen collected 4 weeks after the initial reactive rapid test result. In September 2003, CDC initiated postmarketing surveillance in 14 state and local health departments to monitor the performance of the OraQuick® test. Follow-up was attempted for all persons with reactive OraQuick® tests who had either nonreactive EIAs or negative or indeterminate WB or IFA results. For the 21 such persons who were identified through the surveillance system (Table), follow-up testing was initiated at the testing sites' reference laboratories only as a result of postmarketing surveillance; test results are available for 13 of these persons. At least five HIV-infected persons were informed incorrectly that their rapid HIV test results were false-positive. Several public health and commercial laboratories contacted during this same period also indicated that they did not perform WB or IFA on OraQuick®-reactive specimens if the laboratory EIA was nonreactive. Additional persons might have received erroneous results from incomplete confirmatory testing. CDC emphasizes that reactive rapid HIV tests must be confirmed with WB or IFA, even if a subsequent EIA is nonreactive. If such confirmatory testing yields negative or indeterminate results, follow-up testing should be performed on a blood specimen collected 4 weeks after the initial reactive rapid HIV test result. Reference
Table 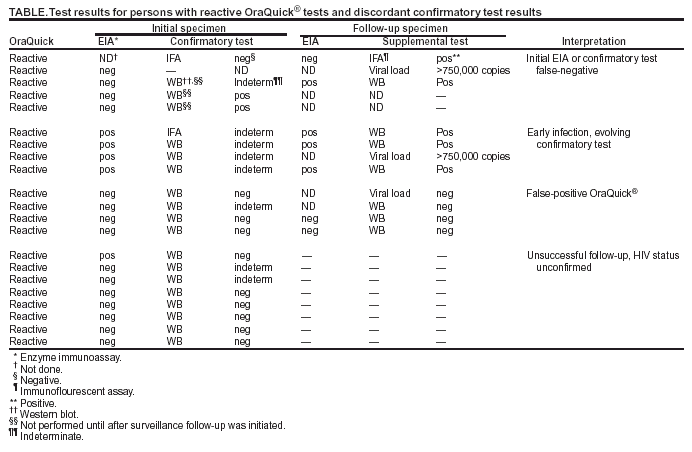 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/18/2004 |
|||||||||
This page last reviewed 3/18/2004
|