Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Measles --- United States, January--May 20, 2011
On May 24, this report was posted as an MMWR Early Release on the MMWR website (http://www.cdc.gov/mmwr).
Measles is a highly contagious, acute viral illness that can lead to serious complications and death. Endemic or sustained measles transmission has not occurred in the United States since the late 1990s, despite continued importations (1). During 2001--2008, a median of 56 (range: 37--140) measles cases were reported to CDC annually (2); during the first 19 weeks of 2011, 118 cases of measles were reported, the highest number reported for this period since 1996. Of the 118 cases, 105 (89%) were associated with importation from other countries, including 46 importations (34 among U.S. residents traveling abroad and 12 among foreign visitors). Among those 46 cases, 40 (87%) were importations from the World Health Organization (WHO) European and South-East Asia regions. Of the 118, 105 (89%) patients were unvaccinated. Forty-seven (40%) patients were hospitalized and nine had pneumonia. The increased number of measles importations into the United States this year underscores the importance of vaccination to prevent measles and its complications.
Measles cases are reported by state health departments to CDC, and confirmed cases are reported via the National Notifiable Disease Surveillance System (NNDSS) using standard case definitions (3). Cases are considered internationally imported if at least some of the exposure period (7--21 days before rash onset) occurred outside the United States and rash occurred within 21 days of entry into the United States, with no known exposure to measles in the United States during that time. Import-associated cases include 1) internationally imported cases; 2) cases that are related epidemiologically to imported cases; and 3) imported virus cases for which an epidemiologic link has not been identified but the viral genotype detected suggests recent importation.* Laboratory confirmation of measles is made by detection in serum of measles-specific immunoglobulin M antibodies, isolation of measles virus, or detection of measles virus RNA by nucleic acid amplification in an appropriate clinical specimen (e.g., nasopharyngeal/oropharyngeal swabs, nasal aspirates, throat washes, or urine). For this report, persons with reported unknown or undocumented vaccination status are considered unvaccinated. An outbreak of measles is defined as a chain of transmission with three or more confirmed cases.
During January 1--May 20, 2011, a total of 118 cases were reported from 23 states and New York City (Figure 1), the highest reported number for the same period since 1996 (Figure 2). Patients ranged in age from 3 months to 68 years; 18 (15%) were aged <12 months, 24 (20%) were aged 1--4 years, 23 (19%) were aged 5--19 years, and 53 (45%) were aged ≥20 years. Measles was laboratory-confirmed in 105 (89%) cases, and measles virus RNA was detected in 52 (44%) cases. Among the 118 cases, 105 (89%) were import-associated, of which 46 (44%) were importations from at least 15 countries (Table), 49 (47%) were import-linked, and 10 (10%) were imported virus cases. The source of 13 cases not import-associated could not be determined. Among the 46 imported cases, most were among persons who acquired the disease in the WHO European Region (20) or South-East Asia Region (20), and 34 (74%) occurred in U.S. residents traveling abroad.
Of the 118 cases, 47 (40%) resulted in hospitalization. Nine patients had pneumonia, but none had encephalitis and none died. All but one hospitalized patient were unvaccinated. The vaccinated patient reported having received 1 dose of measles-containing vaccine and was hospitalized for observation only. Hospitalization rates were highest among infants and children aged <5 years (52%), but rates also were high among children and adults aged ≥5 years (33%).
Unvaccinated persons accounted for 105 (89%) of the 118 cases. Among the 45 U.S. residents aged 12 months−19 years who acquired measles, 39 (87%) were unvaccinated, including 24 whose parents claimed a religious or personal exemption and eight who missed opportunities for vaccination. Among the 42 U.S. residents aged ≥20 years who acquired measles, 35 (83%) were unvaccinated, including six who declined vaccination because of philosophical objections to vaccination. Of the 33 U.S. residents who were vaccine-eligible and had traveled abroad, 30 were unvaccinated and one had received only 1 of the 2 recommended doses.
Nine outbreaks accounted for 58 (49%) of the 118 cases. The median outbreak size was four cases (range: 3--21). In six outbreaks, the index case acquired measles abroad; the source of the other three outbreaks could not be determined. Transmission occurred in households, child care centers, shelters, schools, emergency departments, and at a large community event. The largest outbreak occurred among 21 persons in a Minnesota population in which many children were unvaccinated because of parental concerns about the safety of measles, mumps, and rubella (MMR) vaccine. That outbreak resulted in exposure to many persons and infection of at least seven infants too young to receive MMR vaccine (4).
Reported by
Div of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC. Corresponding contributor: Huong McLean, hmclean@cdc.gov, 404-639-7714.
Editorial Note
As a result of high vaccination coverage, measles elimination (i.e., the absence of endemic transmission) was achieved in the United States in the late 1990s (1) and likely in the rest of the Americas since the early 2000s (5). However, as long as measles remains endemic in the rest of the world, importations into the Western Hemisphere will continue.
The unusually large number of importations into the United States in the first 19 weeks of 2011 is related to recent increases in measles in countries visited by U.S. travelers. The most frequent sources of importation in 2011 were countries in the WHO European Region, which has accounted for the majority of measles importations in the United States since 2005 (2), and the South-East Asia Region. This year, 33 countries in the WHO European Region have reported an increase in measles. France, the source of most of the importations from the European Region, is experiencing a large outbreak, with approximately 10,000 cases reported during the first 4 months of 2011, including 12 cases of encephalitis, a complication that often results in permanent neurologic sequelae, 360 cases of severe measles pneumonia, and six measles-related deaths (6).
Measles can be severe and is highly infectious; following exposure, up to 90% of susceptible persons develop measles. Measles can lead to life-threatening complications. During 1989--1991, a resurgence of measles in the United States resulted in >100 deaths among >55,000 cases reported, reminding U.S. residents of the potential severity of measles, even in the era of modern medical care (7). In the years that followed, the United States witnessed the return of subacute sclerosing panencephalitis among U.S. children, a rare, fatal neurologic complication of measles that had all but disappeared after measles vaccine was introduced in the 1960s (8).
Children and adults who remain unvaccinated and develop measles also put others in their community at risk. For infants too young for routine vaccination (age <12 months) and persons with medical conditions that contraindicate measles immunization, the risk for measles complications is particularly high. These persons depend on high MMR vaccination coverage among those around them to protect them from exposure. In the United States this year, infants aged <12 months accounted for 15% of cases and 15% of hospitalizations. In Europe in recent years, measles has been fatal for several children and adolescents, including some who could not be vaccinated because they were immune compromised.
Rapid control efforts by state and local public health agencies, which are both time intensive and costly, have been a key factor in limiting the size of outbreaks and preventing the spread of measles into communities with increased numbers of unvaccinated persons. Nonetheless, maintenance of high 2-dose MMR vaccination coverage is the most critical factor for sustaining elimination. For measles, even a small decrease in coverage can increase the risk for large outbreaks and endemic transmission, as occurred in the United Kingdom in the past decade (9).
Because of ongoing importations of measles to the United States, health-care providers should suspect measles in persons with a febrile rash illness and clinically compatible symptoms (e.g., cough, coryza, and/or conjunctivitis) who have recently traveled abroad or have had contact with travelers. Providers should isolate and report suspected measles cases immediately to their local health department and obtain specimens for measles testing, including viral specimens for confirmation and genotyping.
MMR vaccine is safe and highly effective in preventing measles and its complications. MMR vaccine is recommended routinely for all children at age 12--15 months, with a second dose at age 4--6 years. For adults with no evidence of immunity to measles,† 1 dose of MMR vaccine is recommended unless the adult is in a high-risk group (i.e., health care personnel, international travelers, or students at post-high school educational institutions), in which case, 2 doses of MMR vaccine are recommended. Measles is endemic in many countries, and exposures might occur in airports and in countries of travel. All travelers aged ≥6 months are eligible to receive MMR vaccine and should be vaccinated before travel (10). Maintaining high immunization rates with MMR vaccine is the cornerstone of outbreak prevention.
Acknowledgments
The findings in this report are based, in part, on contributions by Mary McCauley and Paul Chenoweth, National Center for Immunization and Respiratory Diseases, CDC.
References
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16--17 March 2000. J Infect Dis 2004;189(Suppl 1):S43--7.
- Parker Fiebelkorn A, Redd SB, Gallagher K, et al. Measles in the United States during the postelimination era. J Infect Dis 2010;202:1520--8.
- CDC. Manual for the surveillance of vaccine-preventable diseases. 4th ed. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. Available at http://www.cdc.gov/vaccines/pubs/surv-manual/default.htm. Accessed May 20, 2011.
- CDC. Measles outbreak---Hennepin County, Minnesota, February--March 2011. MMWR 2011;60:421.
- World Health Organization. Global elimination of measles---report by the Secretariate, 16 April 2009. Available at http://apps.who.int/gb/ebwha/pdf_files/EB125/B125_4-en.pdf. Accessed May 20, 2011.
- Institut de Veille Sanitaire. Epidémie de rougeole en France; Actualisation des données au 20 mai 2011. Available at http://www.invs.sante.fr/surveillance/rougeole/Point_rougeole_200511.pdf. Accessed May 23, 2011.
- Gindler J, Tinker S, Markowitz L, et al. Acute measles mortality in the United States, 1987--2002. J Infect Dis 2004;189(Suppl 1):S69--77.
- Bellini WJ, Rota JS, Lowe LE, et al. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis 2005;192:1686--93.
- Editorial team. Measles once again endemic in the United Kingdom. Eurosurveillance 2008;13. Available at http://www.eurosurveillance.org/viewarticle.aspx?articleId=18919. Accessed May 20, 2011.
- Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella--vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998;47(No. RR-8).
What is already known on this topic?
Measles, mumps, and rubella (MMR) vaccine is highly effective in preventing measles and its complications. Sustained measles transmission was eliminated from the United States in the late 1990s, but the disease remains common in many countries globally, and cases of measles are imported into the United States regularly.
What is added by this report?
During the first 19 weeks of 2011, 118 cases of measles were reported in the United States, the highest number for the same period in any year since 1996, and hospitalization rates were high (40%). Importations accounted for 46 (40%) cases, including 34 (74%) cases among U.S. residents who had recently traveled abroad, among 105 import-associated cases.
What are the implications for public health practice?
High 2-dose MMR vaccine coverage is critical for decreasing the risk for reestablishment of endemic measles transmission after importation of measles into the United States. Before any international travel, infants aged 6--11 months should receive 1 dose of MMR vaccine and persons aged ≥12 months should receive 2 doses of MMR vaccine at least 28 days apart or have other evidence of immunity to measles.
FIGURE 1. Distribution and origin of reported measles cases (N = 118) --- United States, January 1--May 20, 2011
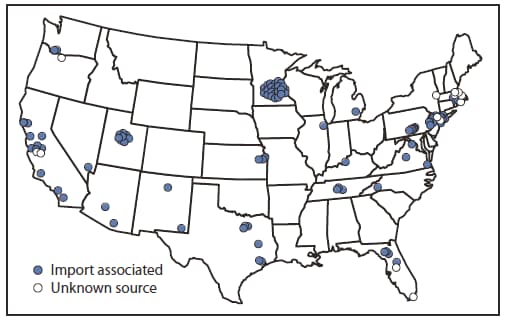
Alternate Text: The figure above shows the distribution and origin of reported measles cases (N = 118) in the United States during January 1-May 20, 2011.
FIGURE 2. Cumulative number of measles cases reported, by month of rash onset --- United States, 2001--2011
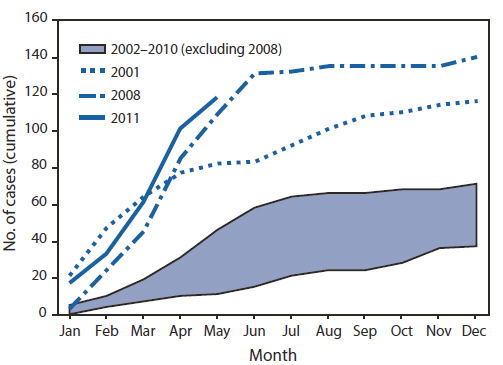
Alternate Text: The figure above shows the cumulative number of measles cases reported, by month of rash onset, in the United States during 2001-2011. During January 1-May 20, 2011, a total of 118 cases were reported, the highest number reported for the same period since 1996.
|
WHO region
|
No. of cases
|
Country
|
No. of cases
|
|
African
|
2
|
Kenya
|
1
|
|
|
Nigeria
|
1
|
|
Eastern Mediterranean
|
2
|
Pakistan
|
1
|
|
|
Jordan
|
1
|
|
European
|
20
|
France
|
11
|
|
|
France/United Kingdom
|
1*
|
|
|
France/Italy/Spain/Germany
|
1*
|
|
|
Italy
|
1
|
|
|
Poland
|
1
|
|
|
Romania
|
1
|
|
|
Spain
|
1
|
|
|
United Kingdom
|
3
|
|
Americas
|
1
|
Dominican Republic
|
1†
|
|
South-East Asia
|
20
|
India
|
14
|
|
|
Indonesia
|
1
|
|
|
Philippines
|
4
|
|
|
Philippines/Vietnam/Singapore/Malaysia
|
1*
|
|
Western Pacific
|
1
|
China
|
1
|




