 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
| Weekly |
| January 17, 1997 / 46(02);25-29 |
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Evaluation of Blunt Suture Needles in Preventing Percutaneous Injuries Among Health-Care Workers During Gynecologic Surgical Procedures -- New York City, March 1993-June 1994Infections with bloodborne pathogens resulting from exposures to blood through percutaneous injuries (PIs) (e.g., needlestick injuries and cuts with sharp objects) are an occupational hazard for health-care workers (HCWs) (1). PIs have been reported during 1%-15% of surgical procedures, mostly associated with suturing (1,2). Most suturing is done using curved suture needles, although straight needles are used by some surgeons for suturing skin. Blunt suture needles (curved suture needles that have a relatively blunt tip) may be less likely to cause PIs because they do not easily penetrate skin. Based on small studies and anecdotal experience, blunt suture needles appear able to replace conventional curved suture needles for suturing many tissues, although they may require more pressure to penetrate the tissues (3-6). This report summarizes results of a study in which CDC collaborated with three teaching hospitals in New York City during 1993-1994 to evaluate a safety device (a blunt suture needle) in gynecologic surgery. The findings indicate that use of blunt needles was associated with statistically significant reductions in PI rates, minimal clinically apparent adverse effects on patient care, and general acceptance by gynecologic surgeons in these hospitals. * Blunt suture needles (Ethiguard{trademark}, Ethicon, Inc., Somerville, New Jersey) ** were evaluated as a potential replacement for conventional curved needles in gynecologic surgery, a specialty in which high PI rates have been reported (2). From March 1993 through June 1994, trained nurse observers at the three hospitals systematically recorded information about the nature and frequency of all PIs and the number and type of suture needles used during gynecologic surgical procedures (laparoscopy and dilation and curettage procedures were excluded from the study). PIs observed or reported during surgery were confirmed by inspection of HCWs' hands before they left the operating room. Beginning in February 1994, hospital investigators replaced conventional curved suture needles with blunt needles on all gynecologic surgical instrument trays; however, surgeons retained the option of requesting conventional needles. During March 1993-June 1994, a total of 1464 gynecologic surgery procedures were observed; of these, 1062 (73%) were performed using only conventional curved needles, 55 (4%) using only blunt needles, and 347 (24%) using both. Straight needles were used in addition to curved needles in 104 procedures. Overall, 87 PIs occurred during 84 (6%) of the 1464 procedures; of these, 61 (70%) involved suture needles, and 26 (30%) involved other surgical devices. Of the 61 injuries involving suture needles, 56 (92%) were associated with conventional curved needles, none with blunt needles, and five (8%) with straight needles. The mean number of curved suture needles used per procedure (24 needles) was constant throughout the study period. The percentage of blunt needles used during a calendar quarter increased, from less than 1% to 55% during the study; during April-June 1994, at least one blunt suture needle was used in 243 (81%) of 299 procedures. The increase in use of blunt suture needles was temporally associated with a decrease in PIs from curved suture needles, from 5.9 PIs per 100 procedures (49 PIs among 835 procedures) in 1993 to 1.1 PIs per 100 procedures (seven PIs among 629 procedures) in 1994 (p less than 0.01) (Figure_1). Rates of PIs with devices other than curved suture needles remained constant (2.1 PIs per 100 procedures). The rates of PIs associated with use of curved suture needles were 1.9 per 1000 conventional curved suture needles used (56 PIs among 28,880 conventional curved suture needles used) and zero per 1000 blunt suture needles used (0 PIs among 6139 blunt suture needles used) (p less than 0.01; relative risk=0.0; 95% confidence interval {CI}=0-0.03). For straight suture needles, the PI rate was 14.2 PIs per 1000 needles used (five PIs among 351 needles used). A logistic regression model was developed to identify and control for potential risk factors for PI during a procedure, including type and duration of the procedure, selected aspects of surgical technique (e.g., using fingers to hold tissue being sutured), estimated patient blood loss, number and type of curved suture needles used, status of the primary surgeon (attending or resident), and whether the primary surgeon had participated in a training program on PI prevention. The model indicated that the use of blunt needles was protective: for each percentage point increase in blunt needles used during a procedure, the adjusted odds ratio for risk of curved suture needle injury was 0.96 (95% CI=0.92-0.98; p less than 0.01). For example, if the percentage of blunt needles used increased from 30% to 40%, the odds of a PI with a curved suture needle were reduced by 34% (i.e., 100 X {1-0.9610}). According to the model, the estimated odds of a PI with a curved suture needle were reduced by 87% when 50% of the suture needles used during a procedure were blunt. In 25 (6%) of the 402 procedures during which blunt needles were used, surgeons reported technical difficulties with the blunt needles, including problems penetrating tissue (18), tearing of tissue (three), needle slippage (three), and bleeding when the needle entered the tissue (one). However, none of these were reported to be clinically important; for procedures performed with and without blunt needles, mean blood loss was similar (328 cc and 351 cc, respectively; p=0.29), and mean operative time was similar (102 min and 106 min, respectively; p=0.24). Long-term complications (e.g., surgical site infections) were not assessed. Reported by: M Mendelson, MD, R Sperling, MD, M Brodman, MD, P Dottino, MD, J Morrow, MD, J Solomon, MPH, Mt. Sinai Medical Center; B Raucher, MD, J Stein, MD, N Roche, MD, A Jacobs, MD, Beth Israel Medical Center; P Nicholas, MD, I Karmin, MD, B Brown, MD, Elmhurst Hospital, New York, New York. Hospital Infections Program, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: The findings in this investigation indicate that in the three participating hospitals, use of blunt suture needles effectively reduced suture-related PIs during gynecologic surgical procedures. Smaller studies in other surgical specialities also concluded that use of blunt suture needles was not associated with PIs (3-6). Although some tissues cannot tolerate the increased force required to use a blunt needle, a blunt needle probably could be substituted for a conventional curved needle in a variety of procedures (3-6). Blunt suture needles may be particularly useful in preventing PIs during suturing in a poorly visualized anatomic space -- a situation associated with increased risks for PI for surgeons and with transmission of hepatitis B virus from surgeons to patients (7). Blunt needles recently have become available in a variety of sizes and suture materials; the effectiveness of blunt needles in reducing PIs suggests that they should be considered for more widespread use in surgical procedures. In this study, the PI rate for straight suture needles was more than seven times the rate associated with conventional curved needles. Straight needles are used by some surgeons to close the skin; however, because safer alternatives (e.g., staplers, conventional curved needles, and possibly blunt needles {6}) are available, indications and techniques for using straight suture needles should be reevaluated. Safety devices designed to reduce the risk for PI to HCWs should not adversely affect patients. In this study, no clinically important patient-care complications attributable to blunt needles were reported by surgeons or suggested based on objective clinical parameters. One limitation of this assessment was the lack of systematic long-term follow-up of patients to assess possible delayed complications of surgery (e.g., surgical-site infections); however, a previously published report on a small number of patients did not document infections in association with use of blunt needles (6). Safety devices must be acceptable to the HCWs who use them. In this and previous reports, blunt needles were acceptable to surgeons as replacement for some or all conventional curved needles in a variety of procedures (3-5). Although specific uses and limitations of blunt needles require further delineation, the findings of this report support the use of blunt needles as an effective component of a PI-prevention program in gynecologic surgery and possibly for other surgical specialties. The Public Health Service is continuing to evaluate the implications of these findings, data from a companion report on safety devices for phlebotomy (8), and other information to assess the need for further guidance on selection, implementation, and evaluation of safety devices in health-care settings. References
* Single copies of this report will be available free until January 16, 1998, from the CDC National AIDS Clearinghouse, P.O. Box 6003, Rockville, MD 20849-6003; telephone (800) 458-5231 or (301) 217-0023. ** Use of trade names and commercial sources is for identification only and does not imply endorsement by the Public Health Service or the U.S. Department of Health and Human Services. Figure_1 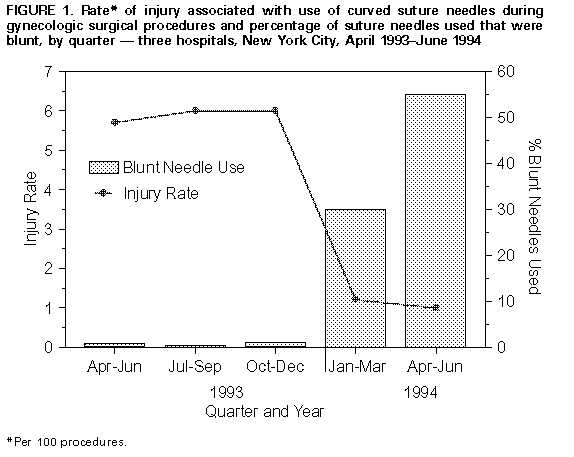 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|