NOTE: The above recommendations must be read along with the footnotes on pages 6–8.
Advisory Committee on Immunization Practices (ACIP) Recommended Immunization Schedules for Persons Aged 0 Through 18 years and Adults Aged 19 Years and Older — United States, 2013
Introduction
Each year, recommendations for routine use of vaccines in children, adolescents, and adults in the United States are developed by the Advisory Committee on Immunization Practices (ACIP). This year, for the first time, recommended immunization schedules for persons aged 0 through 18 years and adults aged 19 years and older are being published together.
Placing These Schedules on Your Website
CDC's National Center for Immunization and Respiratory Diseases (NCIRD) maintains the most current immunization schedules on the Vaccines and Immunizations pages of CDC's website (http://www.cdc.gov/vaccines/schedules), including the schedules published in this supplement. If errors or omissions are discovered after publication of the schedules, CDC posts revised versions on the Vaccines and Immunizations Web pages.
CDC encourages organizations that have previously relied on copying and posting PDFs of the schedules to their websites to instead use a safer method to consistently display current schedules. This form of "content syndication" ensures that the most current and accurate immunization schedule information is on each organization's website. This one-time step assures that your website displays current yearly schedules as soon as they are published, or revised.
To place the schedules on a website, organizations simply include two lines of CDC-furnished computer code on their Web page. Each organization's Web developer places the code into their existing website; the code automatically loads the current CDC schedule and footnotes. The schedule is visible within the organization's Web page, and all other images and Web navigation display unchanged. Any CDC revisions or updates will automatically and immediately be reflected on the organization's Web page.
This form of content syndication also gives organizations the ability to offer a PDF of each schedule on their website. Staff members and Web visitors can print as well as view immunization schedules and be confident they have the most current versions. Instructions for copying and placing syndication code are available at http://www.cdc.gov/vaccines/schedules/hcp/syndicate.html.
CDC offers technical assistance for organizations implementing this form of content syndication. For assistance, readers can complete the e-mail form on the NCIRD Web support page (http://www.cdc.gov/vaccines/web-support.html), and a NCIRD Web team staff member will contact them and provide assistance.
ACIP is chartered as a federal advisory committee to provide expert external advice and guidance to the Director of the Centers for Disease Control and Prevention (CDC) on use of vaccines and related agents for the control of vaccine-preventable diseases in the civilian population of the United States. Recommendations for routine use of vaccines in children and adolescents are harmonized to the greatest extent possible with recommendations made by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American College of Obstetrics and Gynecology (ACOG). Recommendations for routine use of vaccines in adults are reviewed and approved by the American College of Physicians (ACP), AAFP, ACOG, and the American College of Nurse-Midwives. ACIP recommendations adopted by the CDC Director become agency guidelines on the date published in the Morbidity and Mortality Weekly Report (MMWR).
Advisory Committee on Immunization Practices (ACIP) Recommended Immunization Schedule for Persons Aged 0 Through 18 Years — United States, 2013
Corresponding contributor: Iyabode Akinsanya-Beysolow, iakinsanyabeysolow@cdc.gov, 404-639-5251.
Each year, the Advisory Committee on Immunization Practices (ACIP) reviews the current recommended immunization schedules for persons aged 0 through 18 years to ensure that the schedule reflects current recommendations for licensed vaccines. In October 2012, ACIP approved the recommended immunization schedules for persons aged 0 through 18 years for 2013, which includes several changes from 2012.
Health-care providers are advised to use both the recommended schedule and the catch-up schedule (Figures 1 and 2) in combination with their footnotes (pages 6–8) and not as stand-alones. For guidance on the use of all the vaccines in the schedules, including contraindications and precautions to use of a vaccine, providers are referred to the respective ACIP vaccine recommendations.
Printable versions of the regular and catch-up schedules are available at http://www.cdc.gov/vaccines/schedules in various formats, including landscape and pocket-sized, in regular paper or laminated versions. A "parent friendly" regular schedule is available at http://www.cdc.gov/vaccines/schedules/easy-to-read/child.html#print.
For 2013, several new references and links to additional information have been added, including one for travel vaccine requirements and recommendations (1). New references also are provided for vaccination of persons with primary and secondary immunodeficiencies. Changes to the previous schedules (2) include the following:
- Figure 1, "Recommended immunization schedule for persons aged 0 through 18 years" replaces "Recommended immunization schedule for persons aged 0 through 6 years" and "Recommended immunization schedule for persons aged 7 through 18 years."
— Wording was added to bars to represent the respective vaccine dose numbers in the series.
— The meningococcal conjugate vaccine (MCV4) purple bar was extended to age 6 weeks, to reflect licensure of Hib-MenCY vaccine.
— The hepatitis A (HepA) vaccine yellow bar was extended to better reflect routine age recommendations for use of HepA vaccine. New green and purple bars were added to reflect hepatitis A vaccine recommendations for older children.
— Abbreviations for influenza vaccine were updated with the anticipation of quadrivalent vaccine for the 2013–14 influenza season.
— Pneumococcal polysaccharide vaccine (PPSV23) was added to Figure 1. - Footnotes were combined and standardized formatting was used to provide recommendations for each vaccine related to routine vaccination, catch-up vaccination, and vaccination of persons with high-risk medical conditions or under special circumstances.
— Meningococcal conjugate vaccine (MCV4) footnotes were updated to reflect recent recommendations (3).
— Tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccine footnotes were updated to reflect recent recommendations (4).
— Influenza vaccine footnotes were updated to provide dosing guidance for children aged 6 months through 8 years for the 2012–13 and 2013–14 influenza seasons (5). - Meningococcal conjugate (MCV4) vaccine minimum ages and intervals were updated in Figure 2, "Catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind—United States, 2013," to reflect licensure of Hib-MenCY vaccine.
References
- CDC. Traveler's health: vaccinations. Atlanta, GA: US Department of Health and Human Services, CDC; 2012. Available at http://wwwnc.cdc.gov/travel/page/vaccinations.htm.
- CDC. Recommended immunization schedules for persons aged 0–18 years—United States, 2012. MMWR 2012;61(5).
- CDC, Advisory Committee on Immunization Practices. Resolution no. 10/12-2. Vaccines to prevent meningococcal disease. Atlanta, GA: US Department of Health and Human Services, CDC, Advisory Committee on Immunization Practices; 2012.
- CDC. Update on use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in pregnant women. Atlanta, GA: US Department of Health and Human Services, CDC.
- CDC, Advisory Committee on Immunization Practices. Resolution no. 10/12-3. Vaccines to prevent influenza. Atlanta, GA: US Department of Health and Human Services, CDC, Advisory Committee on Immunization Practices; 2012. Available at http://www.cdc.gov/vaccines/programs/vfc/downloads/resolutions/1012-3-flu.pdf.
ACIP Childhood/Adolescent Immunization Work Group
Work Group Chair: Renée Jenkins, MD, Washington, D.C. (ACIP)
Work Group Members: Ruth Karron, MD, Baltimore, Maryland (ACIP); Lorry G. Rubin, MD, New Hyde Park, New York (ACIP); H. Cody Meissner, MD, Boston, Massachusetts; Amy B. Middleman, MD, Houston, Texas; Susan Lett, MD, Boston, Massachusetts; Diane Peterson, Saint Paul, Minnesota; Chris Barry, PA-C, Raleigh, North Carolina; Everett Schlamm, MD, Verona, New Jersey; Katie Brewer, MSN, Silver Springs, Maryland; Patricia Stinchfield, MPH, St Paul, Minnesota; Rosemary Spence, MA, Denver, Colorado; Andrew Kroger, MD, Atlanta, Georgia; William L. Atkinson, MD, Harrisonville, Missouri; Jennifer Hamborsky, MPH, MCHES, Atlanta, Georgia.
Work Group Contributors (CDC): Charles Wolfe, Atlanta, Georgia; Donna Weaver, MN, Atlanta, Georgia; JoEllen Wolicki, Atlanta, Georgia; Melissa Barnett, MS, Atlanta, Georgia; Zunera Mirza, MPH, Atlanta, Georgia
Work Group Secretariat (CDC): Iyabode Akinsanya-Beysolow, MD, Atlanta, Georgia.
Figure 1. Recommended immunization schedule for persons aged 0 through 18 years - 2013.
(FOR THOSE WHO FALL BEHIND OR START LATE, SEE THE CATCH-UP SCHEDULE
[FIGURE 2]).
These recommendations must be read with the footnotes that follow. For those who fall behind or start late, provide catch-up vaccination at the earliest opportunity as indicated by the green bars in Figure 1. To determine minimum intervals between doses, see the catch-up schedule (Figure 2). School entry and adolescent vaccine age groups are in bold.
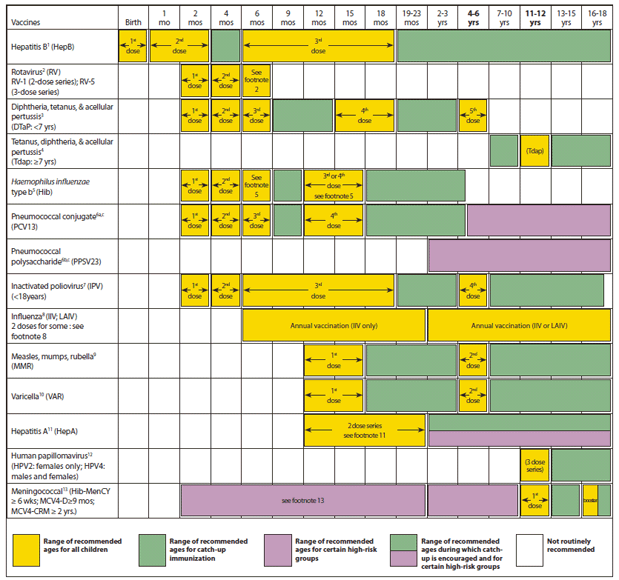
Alternate Text: The figure above shows the recommended immunization schedule for persons aged 0 through 18 years in the United States during 2013. Health-care providers are advised to use both the recommended schedule and the catch-up schedule in combination with their footnotes and not as stand-alones. For Figure 1, "Recommended immunization schedule for persons aged 0 through 18 years" replaces "Recommended immunization schedule for persons aged 0 through 6 years" and "Recommended immunization schedule for persons aged 7 through 18 years."
This schedule includes recommendations in effect as of (month) (day), 2012. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible. The use of a combination vaccine generally is preferred over separate injections of its equivalent component vaccines. Vaccination providers should consult the relevant Advisory Committee on Immunization Practices (ACIP) statement for detailed recommendations, available online at http://www.cdc.gov/vaccines/pubs/acip-list.htm. Clinically significant adverse events that follow vaccination should be reported to the Vaccine Adverse Event Reporting System (VAERS) online (http://www.vaers.hhs.gov) or by telephone (800-822-7967).Suspected cases of vaccine-preventable diseases should be reported to the state or local health department. Additional information, including precautions and contraindications for vaccination, is available from CDC online (http://www.cdc.gov/vaccines) or by telephone (800-CDC-INFO [800-232-4636]).
This schedule is approved by the Advisory Committee on Immunization Practices (www.cdc.gov/vaccines/recs/acip), the American Academy of Pediatrics (www.aap.org), and the American Academy of Family Physicians (www.aafp.org).
NOTE: The above recommendations must be read along with the footnotes on pages 4-5 of this schedule.
FIGURE 2. Catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind — United States, 2013
The figure below provides catch-up schedules and minimum intervals between doses for children whose vaccinations have been delayed. A vaccine series does not need to be restarted, regardless of the time that has elapsed between doses. Use the section appropriate for the child's age. Always use this table in conjunction with Figure 1 and the footnotes that follow.

Alternate Text: The figure above shows the catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind in the United States during 2013. Meningococcal conjugate (MCV4) vaccine minimum ages and intervals were updated in Figure 2, "Catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind-United States, 2013," to reflect licensure of Hib-MenCY vaccine.
1. Hepatitis B (HepB) vaccine. (Minimum age: birth)
Routine vaccination:
At birth
• Administer monovalent HepB vaccine to all newborns before hospital discharge.
• For infants born to hepatitis B surface antigen (HBsAg)–positive mothers, administer HepB vaccine and 0.5 mL of hepatitis B immune globulin (HBIG) within 12 hours of birth. These infants should be tested for HBsAg and antibody to HBsAg (anti-HBs) 1 to 2 months after completion of the HepB series, at age 9 through 18 months (preferably at the next well-child visit).
• If mother's HBsAg status is unknown, within 12 hours of birth administer HepB vaccine to all infants regardless of birth weight. For infants weighing <2,000 grams, administer HBIG in addition to HepB within 12 hours of birth. Determine mother's HBsAg status as soon as possible and, if she is HBsAg-positive, also administer HBIG for infants weighing ≥2,000 grams (no later than age 1 week).
Doses following the birth dose
• The second dose should be administered at age 1 or 2 months. Monovalent HepB vaccine should be used for doses administered before age 6 weeks.
• Infants who did not receive a birth dose should receive 3 doses of a HepB-containing vaccine on a schedule of 0, 1 to 2 months, and 6 months starting as soon as feasible. See Figure 2.
• The minimum interval between dose 1 and dose 2 is 4 weeks and between dose 2 and 3 is 8 weeks. The final (third or fourth) dose in the HepB vaccine series should be administered no earlier than age 24 weeks, and at least 16 weeks after the first dose.
• Administration of a total of 4 doses of HepB vaccine is recommended when a combination vaccine containing HepB is administered after the birth dose.
Catch-up vaccination:
• Unvaccinated persons should complete a 3-dose series.
• A 2-dose series (doses separated by at least 4 months) of adult formulation Recombivax HB is licensed for use in children aged 11 through 15 years.
• For other catch-up issues, see Figure 2.
2. Rotavirus (RV) vaccines. (Minimum age: 6 weeks for both RV-1 [Rotarix] and RV-5 [RotaTeq]).
Routine vaccination:
• Administer a series of RV vaccine to all infants as follows:
1. If RV-1 is used, administer a 2-dose series at 2 and 4 months of age.
2. If RV-5 is used, administer a 3-dose series at ages 2, 4, and 6 months.
3. If any dose in series was RV-5 or vaccine product is unknown for any dose in the series, a total of 3 doses of RV vaccine should be administered.
Catch-up vaccination:
• The maximum age for the first dose in the series is 14 weeks, 6 days.
• Vaccination should not be initiated for infants aged 15 weeks 0 days or older.
• The maximum age for the final dose in the series is 8 months, 0 days.
• If RV-1(Rotarix) is administered for the first and second doses, a third dose is not indicated.
• For other catch-up issues, see Figure 2.
3. Diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine. (Minimum age: 6 weeks)
Routine vaccination:
• Administer a 5-dose series of DTaP vaccine at ages 2, 4, 6, 15–18 months, and 4 through 6 years. The fourth dose may be administered as early as age 12 months, provided at least 6 months have elapsed since the third dose.
Catch-up vaccination:
• The fifth (booster) dose of DTaP vaccine is not necessary if the fourth dose was administered at age 4 years or older.
• For other catch-up issues, see Figure 2.
4. Tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccine. (Minimum age: 10 years for Boostrix, 11 years for Adacel).
Routine vaccination:
• Administer 1 dose of Tdap vaccine to all adolescents aged 11 through 12 years.
• Tdap can be administered regardless of the interval since the last tetanus and diphtheria toxoid-containing vaccine.
• Administer one dose of Tdap vaccine to pregnant adolescents during each pregnancy (preferred during 27 through 36 weeks gestation) regardless of number of years from prior Td or Tdap vaccination.
Catch-up vaccination:
• Persons aged 7 through 10 years who are not fully immunized with the childhood DTaP vaccine series, should receive Tdap vaccine as the first dose in the catch-up series; if additional doses are needed, use Td vaccine. For these children, an adolescent Tdap vaccine should not be given.
• Persons aged 11 through 18 years who have not received Tdap vaccine should receive a dose followed by tetanus and diphtheria toxoids (Td) booster doses every 10 years thereafter.
• An inadvertent dose of DTaP vaccine administered to children aged 7 through 10 years can count as part of the catch-up series. This dose can count as the adolescent Tdap dose, or the child can later receive a Tdap booster dose at age 11–12 years.
• For other catch-up issues, see Figure 2.
5. Haemophilus influenzae type b (Hib) conjugate vaccine. (Minimum age: 6 weeks)
Routine vaccination:
• Administer a Hib vaccine primary series and a booster dose to all infants. The primary series doses should be administered at 2, 4, and 6 months of age; however, if PRP-OMP (PedvaxHib or Comvax) is administered at 2 and 4 months of age, a dose at age 6 months is not indicated. One booster dose should be administered at age 12 through15 months.
• Hiberix (PRP-T) should only be used for the booster (final) dose in children aged 12 months through 4 years, who have received at least 1 dose of Hib.
Catch-up vaccination:
• If dose 1 was administered at ages 12-14 months, administer booster (as final dose) at least 8 weeks after dose 1.
• If the first 2 doses were PRP-OMP (PedvaxHIB or Comvax), and were administered at age 11 months or younger, the third (and final) dose should be administered at age 12 through 15 months and at least 8 weeks after the second dose.
• If the first dose was administered at age 7 through 11 months, administer the second dose at least 4 weeks later and a final dose at age 12 through 15 months, regardless of Hib vaccine (PRP-T or PRP-OMP) used for first dose.
• For unvaccinated children aged 15 months or older, administer only 1 dose.
• For other catch-up issues, see Figure 2.
Vaccination of persons with high-risk conditions:
• Hib vaccine is not routinely recommended for patients older than 5 years of age. However one dose of Hib vaccine should be administered to unvaccinated or partially vaccinated persons aged 5 years or older who have leukemia, malignant neoplasms, anatomic or functional asplenia (including sickle cell disease), human immunodeficiency virus (HIV) infection, or other immunocompromising conditions.
6a. Pneumococcal conjugate vaccine (PCV). (Minimum age: 6 weeks)
Routine vaccination:
• Administer a series of PCV13 vaccine at ages 2, 4, 6 months with a booster at age 12 through 15 months.
• For children aged 14 through 59 months who have received an age-appropriate series of 7-valent PCV (PCV7), administer a single supplemental dose of 13-valent PCV (PCV13).
Catch-up vaccination:
• Administer 1 dose of PCV13 to all healthy children aged 24 through 59 months who are not completely vaccinated for their age.
• For other catch-up issues, see Figure 2.
Vaccination of persons with high-risk conditions:
• For children aged 24 through 71 months with certain underlying medical conditions (see footnote 6c), administer 1 dose of PCV13 if 3 doses of PCV were received previously, or administer 2 doses of PCV13 at least 8 weeks apart if fewer than 3 doses of PCV were received previously.
• A single dose of PCV13 may be administered to previously unvaccinated children aged 6 through 18 years who have anatomic or functional asplenia (including sickle cell disease), HIV infection or an immunocompromising condition, cochlear implant or cerebrospinal fluid leak. See MMWR 2010;59 (No. RR-11), available at http://www.cdc.gov/mmwr/pdf/rr/rr5911.pdf.
• Administer PPSV23 at least 8 weeks after the last dose of PCV to children aged 2 years or older with certain underlying medical conditions (see footnotes 6b and 6c).
6b. Pneumococcal polysaccharide vaccine (PPSV23). (Minimum age: 2 years)
Vaccination of persons with high-risk conditions:
• Administer PPSV23 at least 8 weeks after the last dose of PCV to children aged 2 years or older with certain underlying medical conditions (see footnote 6c). A single revaccination with PPSV should be administered after 5 years to children with anatomic or functional asplenia (including sickle cell disease) or an immunocompromising condition.
6c. Medical conditions for which PPSV23 is indicated in children aged 2 years and older and for which use of PCV13 is indicated in children aged 24 through 71 months:
• Immunocompetent children with chronic heart disease (particularly cyanotic congenital heart disease and cardiac failure); chronic lung disease (including asthma if treated with high-dose oral corticosteroid therapy), diabetes mellitus; cerebrospinal fluid leaks; or cochlear implant.
• Children with anatomic or functional asplenia (including sickle cell disease and other hemoglobinopathies, congenital or acquired asplenia, or splenic dysfunction);
• Children with immunocompromising conditions: HIV infection, chronic renal failure and nephrotic syndrome, diseases associated with treatment with immunosuppressive drugs or radiation therapy, including malignant neoplasms, leukemias, lymphomas and Hodgkin disease; or solid organ transplantation, congenital immunodeficiency.
7. Inactivated poliovirus vaccine (IPV). (Minimum age: 6 weeks)
Routine vaccination:
• Administer a series of IPV at ages 2, 4, 6–18 months, with a booster at age 4–6 years. The final dose in the series should be administered on or after the fourth birthday and at least 6 months after the previous dose.
Catch-up vaccination:
• In the first 6 months of life, minimum age and minimum intervals are only recommended if the person is at risk for imminent exposure to circulating poliovirus (i.e., travel to a polio-endemic region or during an outbreak).
• If 4 or more doses are administered before age 4 years, an additional dose should be administered at age 4 through 6 years.
• A fourth dose is not necessary if the third dose was administered at age 4 years or older and at least 6 months after the previous dose.
• If both OPV and IPV were administered as part of a series, a total of 4 doses should be administered, regardless of the child's current age.
• IPV is not routinely recommended for U.S. residents aged 18 years or older.
• For other catch-up issues, see Figure 2.
8. Influenza vaccines. (Minimum age: 6 months for inactivated influenza vaccine [IIV]; 2 years for live, attenuated influenza vaccine [LAIV])
Routine vaccination:
• Administer influenza vaccine annually to all children beginning at age 6 months. For most healthy, nonpregnant persons aged 2 through 49 years, either LAIV or IIV may be used. However, LAIV should NOT be administered to some persons, including 1) those with asthma, 2) children 2 through 4 years who had wheezing in the past 12 months, or 3) those who have any other underlying medical conditions that predispose them to influenza complications. For all other contraindications to use of LAIV see MMWR 2010; 59 (No. RR-8), available at http://www.cdc.gov/mmwr/pdf/rr/rr5908.pdf.
• Administer 1 dose to persons aged 9 years and older.
For children aged 6 months through 8 years:
• For the 2012–13 season, administer 2 doses (separated by at least 4 weeks) to children who are receiving influenza vaccine for the first time. For additional guidance, follow dosing guidelines in the 2012 ACIP influenza vaccine recommendations, MMWR 2012; 61: 613–618, available at http://www.cdc.gov/mmwr/pdf/wk/mm6132.pdf.
• For the 2013–14 season, follow dosing guidelines in the 2013 ACIP influenza vaccine recommendations.
9. Measles, mumps, and rubella (MMR) vaccine. (Minimum age: 12 months for routine vaccination)
Routine vaccination:
• Administer the first dose of MMR vaccine at age 12 through 15 months, and the second dose at age 4 through 6 years. The second dose may be administered before age 4 years, provided at least 4 weeks have elapsed since the first dose.
• Administer 1 dose of MMR vaccine to infants aged 6 through 11 months before departure from the United States for international travel. These children should be revaccinated with 2 doses of MMR vaccine, the first at age 12 through 15 months (12 months if the child remains in an area where disease risk is high), and the second dose at least 4 weeks later.
• Administer 2 doses of MMR vaccine to children aged 12 months and older, before departure from the United States for international travel. The first dose should be administered on or after age 12 months and the second dose at least 4 weeks later.
Catch-up vaccination:
• Ensure that all school-aged children and adolescents have had 2 doses of MMR vaccine; the minimum interval between the 2 doses is 4 weeks.
10. Varicella (VAR) vaccine. (Minimum age: 12 months)
Routine vaccination:
• Administer the first dose of VAR vaccine at age 12 through 15 months, and the second dose at age 4 through 6 years. The second dose may be administered before age 4 years, provided at least 3 months have elapsed since the first dose. If the second dose was administered at least 4 weeks after the first dose, it can be accepted as valid.
Catch-up vaccination:
• Ensure that all persons aged 7 through 18 years without evidence of immunity (see MMWR 2007;56 [No. RR-4], available at http://www.cdc.gov/mmwr/pdf/rr/rr5604.pdf) have 2 doses of varicella vaccine. For children aged 7 through 12 years the recommended minimum interval between doses is 3 months (if the second dose was administered at least 4 weeks after the first dose, it can be accepted as valid); for persons aged 13 years and older, the minimum interval between doses is 4 weeks.
11. Hepatitis A vaccine (HepA). (Minimum age: 12 months)
Routine vaccination:
• Initiate the 2-dose HepA vaccine series for children aged 12 through 23 months; separate the 2 doses by 6 to 18 months.
• Children who have received 1 dose of HepA vaccine before age 24 months, should receive a second dose 6 to 18 months after the first dose.
• For any person aged 2 years and older who has not already received the HepA vaccine series, 2 doses of HepA vaccine separated by 6 to 18 months may be administered if immunity against hepatitis A virus infection is desired.
Catch-up vaccination:
• The minimum interval between the two doses is 6 months.
Special populations:
• Administer 2 doses of Hep A vaccine at least 6 months apart to previously unvaccinated persons who live in areas where vaccination programs target older children, or who are at increased risk for infection.
12. Human papillomavirus (HPV) vaccines. (HPV4 [Gardasil] and HPV2 [Cervarix]). (Minimum age: 9 years)
Routine vaccination:
• Administer a 3-dose series of HPV vaccine on a schedule of 0, 1-2, and 6 months to all adolescents aged 11-12 years. Either HPV4 or HPV2 may be used for females, and only HPV4 may be used for males.
• The vaccine series can be started beginning at age 9 years.
• Administer the second dose 1 to 2 months after the first dose and the third dose 6 months after the first dose (at least 24 weeks after the first dose).
Catch-up vaccination:
• Administer the vaccine series to females (either HPV2 or HPV4) and males (HPV4) at age 13 through 18 years if not previously vaccinated.
• Use recommended routine dosing intervals (see above) for vaccine series catch-up.
13. Meningococcal conjugate vaccines (MCV). (Minimum age: 6 weeks for Hib-MenCY, 9 months for Menactra [MCV4-D], 2 years for Menveo [MCV4-CRM]).
Routine vaccination:
• Administer MCV4 vaccine at age 11–12 years, with a booster dose at age 16 years.
• Adolescents aged 11 through 18 years with human immunodeficiency virus (HIV) infection should receive a 2-dose primary series of MCV4, with at least 8 weeks between doses. See MMWR 2011; 60:1018–1019 available at: http://www.cdc.gov/mmwr/pdf/wk/mm6030.pdf.
• For children aged 9 months through 10 years with high-risk conditions, see below.
Catch-up vaccination:
• Administer MCV4 vaccine at age 13 through 18 years if not previously vaccinated.
• If the first dose is administered at age 13 through 15 years, a booster dose should be administered at age 16 through 18 years with a minimum interval of at least 8 weeks between doses.
• If the first dose is administered at age 16 years or older, a booster dose is not needed.
• For other catch-up issues, see Figure 2.
Vaccination of persons with high-risk conditions:
• For children younger than 19 months of age with anatomic or functional asplenia (including sickle cell disease), administer an infant series of Hib-MenCY at 2, 4, 6, and 12-15 months.
• For children aged 2 through 18 months with persistent complement component deficiency, administer either an infant series of Hib-MenCY at 2, 4, 6, and 12 through 15 months or a 2-dose primary series of MCV4-D starting at 9 months, with at least 8 weeks between doses. For children aged 19 through 23 months with persistent complement component deficiency who have not received a complete series of Hib-MenCY or MCV4-D, administer 2 primary doses of MCV4-D at least 8 weeks apart.
• For children aged 24 months and older with persistent complement component deficiency or anatomic or functional asplenia (including sickle cell disease), who have not received a complete series of Hib-MenCY or MCV4-D, administer 2 primary doses of either MCV4-D or MCV4-CRM. If MCV4-D (Menactra) is administered to a child with asplenia (including sickle cell disease), do not administer MCV4-D until 2 years of age and at least 4 weeks after the completion of all PCV13 doses. See MMWR 2011;60:1391–2, available at http://www.cdc.gov/mmwr/pdf/wk/mm6040.pdf.
• For children aged 9 months and older who are residents of or travelers to countries in the African meningitis belt or to the Hajj, administer an age appropriate formulation and series of MCV4 for protection against serogroups A and W-135. Prior receipt of Hib-MenCY is not sufficient for children traveling to the meningitis belt or the Hajj. See MMWR 2011;60:1391–2, available at http://www.cdc.gov/mmwr/pdf/wk/mm6040.pdf.
• For children who are present during outbreaks caused by a vaccine serogroup, administer or complete an age and formulation-appropriate series of Hib-MenCY or MCV4.
• For booster doses among persons with high-risk conditions refer to http://www.cdc.gov/vaccines/pubs/acip-list.htm#mening.
Additional Vaccine Information
• For contraindications and precautions to use of a vaccine and for additional information regarding that vaccine, vaccination providers should consult the relevant ACIP statement available online at http://www.cdc.gov/vaccines/pubs/acip-list.htm.
• For the purposes of calculating intervals between doses, 4 weeks = 28 days. Intervals of 4 months or greater are determined by calendar months.
• Information on travel vaccine requirements and recommendations is available at http://wwwnc.cdc.gov/travel/page/vaccinations.htm.
• For vaccination of persons with primary and secondary immunodeficiencies, see Table 13, "Vaccination of persons with primary and secondary immunodeficiencies," in General Recommendations on Immunization (ACIP), available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm; and American Academy of Pediatrics. Passive immunization. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS eds. Red book: 2012 report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics.
Footnotes: Recommended Immunization Schedule for Persons Aged 0 Through 18 Years — United States, 2013
Additional guidance for use of the vaccines described in this publication is available at http://www.cdc.gov/vaccines/pubs/acip-list.htm
Advisory Committee on Immunization Practices (ACIP) Recommended Immunization Schedule for Adults Aged 19 Years and Older — United States, 2013
Corresponding contributor: Carolyn B. Bridges, cbridges@cdc.gov, 404-639-8689.
Vaccines are recommended for adults on the basis of age, prior vaccinations, health conditions, lifestyle, occupation, and travel. Current levels of vaccination coverage among adults are low (1). Health-care providers should be aware of the importance of routinely assessing patients' vaccination histories and recommending and providing routinely recommended vaccines. A strong recommendation from a health-care provider is associated with increased uptake of vaccines (2,3). Other interventions shown to increase vaccine uptake, such as implementation of reminder/recall systems and standing orders, have been summarized by the Community Guide (3).
The Advisory Committee on Immunization Practices (ACIP) annually reviews and updates the adult immunization schedule, which is designed to provide vaccine providers with a summary of existing ACIP recommendations regarding the routine use of vaccines for adults (Figures 1 and 2). The adult schedule also includes a table summarizing the primary contraindications and precautions for routinely recommended vaccines (Table). In October 2012, ACIP approved the adult immunization schedule for 2013. This schedule also incorporates changes to vaccine recommendations voted on by ACIP at its October 24–25, 2012 meeting.
The primary updates include adding information for the first time on the use of 13-valent pneumococcal conjugate vaccine (PCV13) and the timing of administration of PCV13 relative to the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in adults (4). PCV13 is recommended for adults aged 19 years and older with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants. The schedule also clarifies which adults need 1 or 2 doses of PPSV23 before age 65 years. Other changes to the PPSV23 footnote include adding information regarding recommendations for vaccination when vaccination status is unknown.
For tetanus, diphtheria, and acellular pertussis (Tdap) vaccine, recommendations have been expanded to include routine vaccination of adults aged 65 years and older and for pregnant women to receive Tdap vaccine with each pregnancy. The ideal timing of Tdap vaccination during pregnancy is during 27–36 weeks' gestation. This recommendation was made to increase the likelihood of optimal protection for the pregnant woman and her infant during the first few months of the infant's life, when the child is too young for vaccination but at highest risk for severe illness and death from pertussis (5,6).
Manufacturers of the live, attenuated influenza vaccine (LAIV) have obtained Food and Drug Administration (FDA) approval for a quadrivalent influenza vaccine that contains one influenza A (H3N2), one influenza A (H1N1) and two influenza B vaccine virus strains, one from each lineage of circulating influenza B viruses. In approximately half of the recent influenza seasons, the trivalent influenza vaccine has included an influenza B vaccine virus from the lineage different from the predominant circulating influenza B strains (7). Inclusion of both lineages of influenza B virus is intended to increase the likelihood that the vaccine provides cross-reactive antibody against a higher proportion of circulating influenza B viruses. For LAIV, beginning with the 2013–14 season, it is expected that only the quadrivalent formulation will be available and manufacture of the trivalent formulation will cease. It is possible that quadrivalent inactivated influenza vaccine formulations might be available for the 2013–14 season as well. Because a mix of quadrivalent and trivalent influenza vaccines might be available in 2013–14, the abbreviation for inactivated influenza vaccine has been changed from trivalent inactivated influenza vaccine (TIV) to inactivated influenza vaccine (IIV). The abbreviation for LAIV remains unchanged.
Minor wording changes, clarifications, or simplifications have been made to footnotes for measles, mumps, rubella vaccine (MMR), human papillomavirus vaccine (HPV), zoster vaccine, and hepatitis A and hepatitis B vaccines. A correction has been made to Figure 1 for MMR vaccine: the bar that indicated the vaccine might be used in certain situations by persons born before 1957 has been removed. Persons born before 1957 are considered immune, and routine vaccination is not recommended. Considerations for the possible use of MMR vaccine in outbreak situations are included in the 2011 MMWR publication on vaccination of health-care personnel (8). In addition, a correction was made to Figure 2 for PPSV23. This vaccine is indicated for men who have sex with men if they have another risk factor (e.g., age or underlying condition); the bar has been changed from yellow to purple to more accurately reflect the recommendation.
Vaccine providers are reminded to consult the full ACIP vaccine recommendations if they have questions and to bear in mind that additional updates might be made for specific vaccines during the year between updates to the adult schedule. Printable versions of the 2013 adult immunization schedule and other information is available at http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Information about adult vaccination is available at http://www.cdc.gov/vaccines/default.htm. ACIP statements and information for specific vaccines is available at http://www.cdc.gov/vaccines/pubs/acip-list.htm. Adverse events from vaccination should be reported at http://www.vaers.hhs.gov or by telephone, 800-822-7967. This schedule has been approved by the American Academy of Family Physicians, the American College of Physicians, the American College of Obstetrics and Gynecology, and the American College of Nurse-Midwives. The adult immunization schedule is published in the Annals of Internal Medicine at the same time that it is published in MMWR.
Changes for 2013
Footnotes
- Information was added to footnote #1 to direct readers to additional information regarding recommendations for vaccination when vaccination status is unknown.
- The influenza vaccination footnote (#2) now uses the abbreviation IIV for inactivated influenza vaccine and drops the abbreviation TIV for trivalent inactivated vaccine (TIV). For the 2013–14 influenza season, it is expected that the LAIV will be available only in a quadrivalent formulation; IIV might be available in both trivalent and quadrivalent formulations.
- The tetanus, diphtheria, and acellular pertussis (Td/Tdap) vaccination footnote (#3) is updated to include the recommendation to vaccinate pregnant women with Tdap during each pregnancy, regardless of the interval since prior Td/Tdap vaccination and to include the recommendation for all other adults, including persons aged 65 years and older, to receive 1 dose of Tdap vaccine.
- The varicella (#4) and HPV (#5) footnotes were simplified; no changes in recommendations were made. Additional information was added to the HPV footnote regarding HPV vaccination and pregnancy.
- The zoster footnote (#6) was changed to clarify that ACIP recommends vaccination of persons beginning at age 60 years both for persons with and without underlying health conditions for whom the vaccine is not contraindicated.
- The measles, mumps, rubella (MMR) vaccine footnote (#7) was modified to reflect the new recommendation that a provider diagnosis of measles, mumps, or rubella is not considered acceptable evidence of immunity. Previously, a provider diagnosis of measles or mumps, but not rubella, was considered acceptable evidence of immunity.
- Information was added to the pneumococcal polysaccharide (PPSV23) vaccination footnote (#8) and PPSV23 revaccination footnote (#9) to clarify that persons with certain medical conditions are recommended to receive 2 doses of PPSV23 before age 65 years. In addition, even those who receive 2 doses of PPSV23 before age 65 years are recommended to receive PPSV23 at age 65 years, as long as it has been 5 years since the most recent dose. The PPSV23 footnote refers to footnote #10 for pneumococcal conjugate 13-valent vaccine (PCV13) regarding the timing of PCV13 vaccine relative to PPSV23 for those persons recommended to be vaccinated with both pneumococcal vaccines.
- A new footnote (#10) was added for PCV13 vaccine. This vaccine is recommended for adults aged 19 years and older with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants. Those not previously vaccinated with PCV13 or PPSV23 should receive a single dose of PCV13, followed by a dose of PPSV23 at least 8 weeks later. Those previously vaccinated with PPSV23 should be vaccinated with PCV13 one year or more after PPSV23 vaccination (4).
- The hepatitis A vaccine footnote (#12) was updated to clarify that vaccination is recommended for persons with a history of either injection or noninjection illicit drug use.
- The hepatitis B vaccine footnote (#13) includes minor wording changes and adds information on the vaccine schedule for hepatitis B vaccine series for the Recombivax HB vaccine. The dosing schedules for other hepatitis B vaccines were included in prior years' schedules.
Figures
- For figure 1, the bar for Tdap/Td for persons aged 65 years and older has been changed to solid yellow because all adults, including those 65 years and older, are now recommended to receive one dose of Tdap vaccine (5).
- The bar for MMR vaccine for persons born before 1957 has been removed. MMR vaccine is not recommended routinely for persons born before 1957. Considerations for vaccination in measles or mumps outbreak settings are discussed in the ACIP recommendations for health-care personnel (8).
- A new row for PCV13 vaccine has been added.
- For Figure 2, the recommendation for Tdap vaccination with each pregnancy is included, with a single dose of Tdap recommended for all other groups (6).
- A correction was made to change the color for PPSV23 from yellow to purple for men who have sex with men (MSM). PPSV23 is recommended for MSM who have another risk factor such as age group or medical condition.
- A row for PCV13 was added (4).
Contraindications and Precautions Table
- The inactivated influenza vaccine precautions were updated to indicate that persons who experience only hives with exposure to eggs should receive IIV rather than LAIV.
- Pregnancy was removed as a precaution for hepatitis A vaccine. This is an inactivated vaccine, and similar to hepatitis B vaccines, is recommended if another high risk condition or other indication is present.
- Language was clarified regarding the precaution for use of antiviral medications and vaccination with varicella or zoster vaccines.
References
- CDC. Noninfluenza vaccination coverage among adults—United States, 2011. MMWR 2013;62(4).
- CDC. Influenza vaccination coverage among pregnant women—2011–12 influenza season, United States. MMWR 2012;61:758–63.
- Community Preventive Services Task Force. Vaccinations to prevent diseases: universally recommended vaccinations. Available at http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed December 21, 2012.
- CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2012;61:816–9.
- CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older — Advisory Committee on Immunization Practices (ACIP), 2012. MMWR 2012;61:468–70.
- CDC. Update on use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in pregnant women. Atlanta, GA: US Department of Health and Human Services, CDC. Available at http://www.cdc.gov/vaccines/recs/provisional/downloads/tdap-pregnant-oct-2012.pdf. Accessed December 21, 2012.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012;30:1993–8.
- CDC. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. RR-7).
ACIP Adult Immunization Work Group
Work Group Chair: Tamera Coyne-Beasley, MD, Chapel Hill, North Carolina (ACIP.)
Work Group Members: Tammy Clark, Jackson, Mississippi; Kathleen Harriman, PhD, Richmond, California; Molly Howell, MPH, Bismarck, North Dakota; Laura Pinkston Koenigs, MD, Springfield, Massachusetts; Marie-Michele Leger, MPH, Alexandria, VA; Susan M. Lett, MD, Boston, Massachusetts; Robert Palinkas, MD, Urbana, Illinois; Diane Peterson, Saint Paul, Minnesota; Gregory Poland, MD, Rochester, Minnesota; Laura E. Riley, MD, Boston, Massachusetts; William Schaffner, MD, Nashville, Tennessee; Kenneth Schmader, MD, Durham, North Carolina; Litjen Tan, PhD, Chicago, Illinois; Jonathan L. Temte, MD, PhD, Madison, Wisconsin; Richard Zimmerman, MD, Pittsburgh, Pennsylvania.
Work Group Contributors (CDC): Lisa Grohskopf, MD, Craig Hales, MD, Charles LeBaron, MD; Jennifer L. Liang, DVM, Lauri Markowitz, MD; Matthew Moore, MD; Amy Parker Fiebelkorn, MSN, MPH; Sarah Schillie, MD; Raymond A. Strikas, MD, MPH; and Walter W. Williams, MD, Atlanta, Georgia.
Work Group Secretariat (CDC): Carolyn B. Bridges, MD, Atlanta, Georgia
These recommendations must be read with the footnotes that follow.

Alternate Text: The figure above shows recommended adult immunization schedule, by vaccine and age group. For Figure 1, the bar for Tdap/Td for persons aged 65 years and older has been changed to solid yellow because all adults, including those 65 years and older, are now recommended to receive one dose of Tdap vaccine. The bar for MMR vaccine for persons born before 1957 has been removed. MMR vaccine is not recommended routinely for persons born before 1957. Considerations for vaccination in measles or mumps outbreak settings are discussed in the ACIP recommendations for health-care personnel. A new row for PCV13 vaccine has been added.

Alternate Text: The figure above shows recommended vaccinations indicated for adults based on medical and other indications. For Figure 2, the recommendation for Tdap vaccination with each pregnancy is included, with a single dose of Tdap recommended for all other groups. A correction was made to change the color for PPSV23 from yellow to purple for men who have sex with men (MSM). PPSV23 is recommended for MSM who have another risk factor such as age group or medical condition. A row for PCV13 was added.
Footnotes: Recommended Immunization Schedule for Adults Aged 19 Years and Older — United States, 2013
1. Additional information
• Additional guidance for the use of the vaccines described in this supplement is available at http://www.cdc.gov/vaccines/pubs/acip-list.htm.
• Information on vaccination recommendations when vaccination status is unknown and other general immunization information can be found in the General Recommendations on Immunization at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm.
• Information on travel vaccine requirements and recommendations (e.g., for hepatitis A and B, meningococcal, and other vaccines) are available at http://wwwnc.cdc.gov/travel/page/vaccinations.htm.
2. Influenza vaccination
• Annual vaccination against influenza is recommended for all persons aged 6 months and older.
• Persons aged 6 months and older, including pregnant women, can receive the inactivated influenza vaccine (IIV).
• Healthy, nonpregnant persons aged 2–49 years without high-risk medical conditions can receive either intranasally administered live, attenuated influenza vaccine (LAIV) (FluMist), or IIV. Health-care personnel who care for severely immunocompromised persons (i.e., those who require care in a protected environment) should receive IIV rather than LAIV.
• The intramuscularly or intradermally administered IIV are options for adults aged 18–64 years.
• Adults aged 65 years and older can receive the standard dose IIV or the high-dose IIV (Fluzone High-Dose).
3. Tetanus, diphtheria, and acellular pertussis (Td/Tdap) vaccination
• Administer one dose of Tdap vaccine to pregnant women during each pregnancy (preferred during 27–36 weeks' gestation), regardless of number of years since prior Td or Tdap vaccination.
• Administer Tdap to all other adults who have not previously received Tdap or for whom vaccine status is unknown. Tdap can be administered regardless of interval since the most recent tetanus or diphtheria-toxoid containing vaccine.
• Adults with an unknown or incomplete history of completing a 3-dose primary vaccination series with Td-containing vaccines should begin or complete a primary vaccination series including a Tdap dose.
• For unvaccinated adults, administer the first 2 doses at least 4 weeks apart and the third dose 6–12 months after the second.
• For incompletely vaccinated (i.e., less than 3 doses) adults, administer remaining doses.
• Refer to the Advisory Committee on Immunization Practices (ACIP) statement for recommendations for administering Td/Tdap as prophylaxis in wound management (see footnote #1).
4. Varicella vaccination
• All adults without evidence of immunity to varicella (as defined below) should receive 2 doses of single-antigen varicella vaccine or a second dose if they have received only 1 dose.
• Special consideration for vaccination should be given to those who have close contact with persons at high risk for severe disease (e.g., health-care personnel and family contacts of persons with immunocompromising conditions) or are at high risk for exposure or transmission (e.g., teachers; child care employees; residents and staff members of institutional settings, including correctional institutions; college students; military personnel; adolescents and adults living in households with children; nonpregnant women of childbearing age; and international travelers).
• Pregnant women should be assessed for evidence of varicella immunity. Women who do not have evidence of immunity should receive the first dose of varicella vaccine upon completion or termination of pregnancy and before discharge from the health-care facility. The second dose should be administered 4–8 weeks after the first dose.
• Evidence of immunity to varicella in adults includes any of the following:
- documentation of 2 doses of varicella vaccine at least 4 weeks apart;
- U.S.-born before 1980 except health-care personnel and pregnant women;
- history of varicella based on diagnosis or verification of varicella disease by a health-care provider;
- history of herpes zoster based on diagnosis or verification of herpes zoster disease by a health-care provider; or
- laboratory evidence of immunity or laboratory confirmation of disease.
5. Human papillomavirus (HPV) vaccination
• Two vaccines are licensed for use in females, bivalent HPV vaccine (HPV2) and quadrivalent HPV vaccine (HPV4), and one HPV vaccine for use in males (HPV4).
• For females, either HPV4 or HPV2 is recommended in a 3-dose series for routine vaccination at age 11 or 12 years, and for those aged 13 through 26 years, if not previously vaccinated.
• For males, HPV4 is recommended in a 3-dose series for routine vaccination at age 11 or 12 years, and for those aged 13 through 21 years, if not previously vaccinated. Males aged 22 through 26 years may be vaccinated.
• HPV4 is recommended for men who have sex with men (MSM) through age 26 years for those who did not get any or all doses when they were younger.
• Vaccination is recommended for immunocompromised persons (including those with HIV infection) through age 26 years for those who did not get any or all doses when they were younger.
• A complete series for either HPV4 or HPV2 consists of 3 doses. The second dose should be administered 1–2 months after the first dose; the third dose should be administered 6 months after the first dose (at least 24 weeks after the first dose).
• HPV vaccines are not recommended for use in pregnant women. However, pregnancy testing is not needed before vaccination. If a woman is found to be pregnant after initiating the vaccination series, no intervention is needed; the remainder of the 3-dose series should be delayed until completion of pregnancy.
• Although HPV vaccination is not specifically recommended for health-care personnel (HCP) based on their occupation, HCP should receive the HPV vaccine as recommended (see above).
6. Zoster vaccination
• A single dose of zoster vaccine is recommended for adults aged 60 years and older regardless of whether they report a prior episode of herpes zoster. Although the vaccine is licensed by the Food and Drug Administration (FDA) for use among and can be administered to persons aged 50 years and older, ACIP recommends that vaccination begins at age 60 years.
• Persons aged 60 years and older with chronic medical conditions may be vaccinated unless their condition constitutes a contraindication, such as pregnancy or severe immunodeficiency.
• Although zoster vaccination is not specifically recommended for HCP, they should receive the vaccine if they are in the recommended age group.
7. Measles, mumps, rubella (MMR) vaccination
• Adults born before 1957 generally are considered immune to measles and mumps. All adults born in 1957 or later should have documentation of 1 or more doses of MMR vaccine unless they have a medical contraindication to the vaccine, or laboratory evidence of immunity to each of the three diseases. Documentation of provider-diagnosed disease is not considered acceptable evidence of immunity for measles, mumps, or rubella.
Measles component:
• A routine second dose of MMR vaccine, administered a minimum of 28 days after the first dose, is recommended for adults who
- are students in postsecondary educational institutions;
- work in a health-care facility; or
- plan to travel internationally.
• Persons who received inactivated (killed) measles vaccine or measles vaccine of unknown type during 1963–1967 should be revaccinated with 2 doses of MMR vaccine.
Mumps component:
• A routine second dose of MMR vaccine, administered a minimum of 28 days after the first dose, is recommended for adults who
- are students in a postsecondary educational institution;
- work in a health-care facility; or
- plan to travel internationally.
• Persons vaccinated before 1979 with either killed mumps vaccine or mumps vaccine of unknown type who are at high risk for mumps infection (e.g., persons who are working in a health-care facility) should be considered for revaccination with 2 doses of MMR vaccine.
Rubella component:
• For women of childbearing age, regardless of birth year, rubella immunity should be determined. If there is no evidence of immunity, women who are not pregnant should be vaccinated. Pregnant women who do not have evidence of immunity should receive MMR vaccine upon completion or termination of pregnancy and before discharge from the health-care facility.
HCP born before 1957:
• For unvaccinated health-care personnel born before 1957 who lack laboratory evidence of measles, mumps, and/or rubella immunity or laboratory confirmation of disease, health-care facilities should consider vaccinating personnel with 2 doses of MMR vaccine at the appropriate interval for measles and mumps or 1 dose of MMR vaccine for rubella.
8. Pneumococcal polysaccharide (PPSV23) vaccination
• Vaccinate all persons with the following indications:
- all adults aged 65 years and older;
- adults younger than age 65 years with chronic lung disease (including chronic obstructive pulmonary disease, emphysema, and asthma); chronic cardiovascular diseases; diabetes mellitus; chronic renal failure; nephrotic syndrome; chronic liver disease (including cirrhosis); alcoholism; cochlear implants; cerebrospinal fluid leaks; immunocompromising conditions; and functional or anatomic asplenia (e.g., sickle cell disease and other hemoglobinopathies, congenital or acquired asplenia, splenic dysfunction, or splenectomy [if elective splenectomy is planned, vaccinate at least 2 weeks before surgery]);
- residents of nursing homes or long-term care facilities; and
- adults who smoke cigarettes.
• Persons with immunocompromising conditions and other selected conditions are recommended to receive PCV13 and PPSV23 vaccines. See footnote #10 for information on timing of PCV13 and PPSV23 vaccinations.
• Persons with asymptomatic or symptomatic HIV infection should be vaccinated as soon as possible after their diagnosis.
• When cancer chemotherapy or other immunosuppressive therapy is being considered, the interval between vaccination and initiation of immunosuppressive therapy should be at least 2 weeks. Vaccination during chemotherapy or radiation therapy should be avoided.
• Routine use of PPSV23 is not recommended for American Indians/Alaska Natives or other persons younger than age 65 years unless they have underlying medical conditions that are PPSV23 indications. However, public health authorities may consider recommending PPSV23 for American Indians/Alaska Natives who are living in areas where the risk for invasive pneumococcal disease is increased.
• When indicated, PPSV23 should be administered to patients who are uncertain of their vaccination status and there is no record of previous vaccination. When PCV13 is also indicated, a dose of PCV13 should be given first (see footnote #10).
9. Revaccination with PPSV23
• One-time revaccination 5 years after the first dose is recommended for persons aged 19 through 64 years with chronic renal failure or nephrotic syndrome; functional or anatomic asplenia (e.g., sickle cell disease or splenectomy); and for persons with immunocompromising conditions.
• Persons who received 1 or 2 doses of PPSV23 before age 65 years for any indication should receive another dose of the vaccine at age 65 years or later if at least 5 years have passed since their previous dose.
• No further doses are needed for persons vaccinated with PPSV23 at or after age 65 years.
10. Pneumococcal conjugate 13-valent vaccination (PCV13)
• Adults aged 19 years or older with immunocompromising conditions (including chronic renal failure and nephrotic syndrome), functional or anatomic asplenia, CSF leaks or cochlear implants, and who have not previously received PCV13 or PPSV23 should receive a single dose of PCV13 followed by a dose of PPSV23 at least 8 weeks later.
• Adults aged 19 years or older with the aforementioned conditions who have previously received one or more doses of PPSV23 should receive a dose of PCV13 one or more years after the last PPSV23 dose was received. For those that require additional doses of PPSV23, the first such dose should be given no sooner than 8 weeks after PCV13 and at least 5 years since the most recent dose of PPSV23.
• When indicated, PCV13 should be administered to patients who are uncertain of their vaccination status history and there is no record of previous vaccination.
• Although PCV13 is licensed by the Food and Drug Administration (FDA) for use among and can be administered to persons aged 50 years and older, ACIP recommends PCV13 for adults aged 19 years and older with the specific medical conditions noted above.
11. Meningococcal vaccination
• Administer 2 doses of meningococcal conjugate vaccine quadrivalent (MCV4) at least 2 months apart to adults with functional asplenia or persistent complement component deficiencies.
• HIV-infected persons who are vaccinated also should receive 2 doses.
• Administer a single dose of meningococcal vaccine to microbiologists routinely exposed to isolates of Neisseria meningitidis, military recruits, and persons who travel to or live in countries in which meningococcal disease is hyperendemic or epidemic.
• First-year college students up through age 21 years who are living in residence halls should be vaccinated if they have not received a dose on or after their 16th birthday.
• MCV4 is preferred for adults with any of the preceding indications who are aged 55 years and younger; meningococcal polysaccharide vaccine (MPSV4) is preferred for adults aged 56 years and older.
• Revaccination with MCV4 every 5 years is recommended for adults previously vaccinated with MCV4 or MPSV4 who remain at increased risk for infection (e.g., adults with anatomic or functional asplenia or persistent complement component deficiencies).
12. Hepatitis A vaccination
• Vaccinate any person seeking protection from hepatitis A virus (HAV) infection and persons with any of the following indications:
- men who have sex with men and persons who use injection or noninjection illicit drugs;
- persons working with HAV-infected primates or with HAV in a research laboratory setting;
- persons with chronic liver disease and persons who receive clotting factor concentrates;
- persons traveling to or working in countries that have high or intermediate endemicity of hepatitis A; and
- unvaccinated persons who anticipate close personal contact (e.g., household or regular babysitting) with an international adoptee during the first 60 days after arrival in the United States from a country with high or intermediate endemicity. (See footnote #1 for more information on travel recommendations). The first dose of the 2-dose hepatitis A vaccine series should be administered as soon as adoption is planned, ideally 2 or more weeks before the arrival of the adoptee.
• Single-antigen vaccine formulations should be administered in a 2-dose schedule at either age 0 and 6–12 months (Havrix), or age 0 and 6–18 months (Vaqta). If the combined hepatitis A and hepatitis B vaccine (Twinrix) is used, administer 3 doses at 0, 1, and 6 months; alternatively, a 4-dose schedule may be used, administered on days 0, 7, and 21–30, followed by a booster dose at month 12.
13. Hepatitis B vaccination
• Vaccinate persons with any of the following indications and any person seeking protection from hepatitis B virus (HBV) infection:
- sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., persons with more than one sex partner during the previous 6 months); persons seeking evaluation or treatment for a sexually transmitted disease (STD); current or recent injection-drug users; and men who have sex with men;
- health-care personnel and public-safety workers who are potentially exposed to blood or other infectious body fluids;
- persons with diabetes younger than age 60 years as soon as feasible after diagnosis; persons with diabetes who are age 60 years or older at the discretion of the treating clinician based on increased need for assisted blood glucose monitoring in long-term care facilities, likelihood of acquiring hepatitis B infection, its complications or chronic sequelae, and likelihood of immune response to vaccination;
- persons with end-stage renal disease, including patients receiving hemodialysis; persons with HIV infection; and persons with chronic liver disease;
- household contacts and sex partners of hepatitis B surface antigen-positive persons; clients and staff members of institutions for persons with developmental disabilities; and international travelers to countries with high or intermediate prevalence of chronic HBV infection; and
- all adults in the following settings: STD treatment facilities; HIV testing and treatment facilities; facilities providing drug-abuse treatment and prevention services; health-care settings targeting services to injection-drug users or men who have sex with men; correctional facilities; end-stage renal disease programs and facilities for chronic hemodialysis patients; and institutions and nonresidential daycare facilities for persons with developmental disabilities.
• Administer missing doses to complete a 3-dose series of hepatitis B vaccine to those persons not vaccinated or not completely vaccinated. The second dose should be administered 1 month after the first dose; the third dose should be given at least 2 months after the second dose (and at least 4 months after the first dose). If the combined hepatitis A and hepatitis B vaccine (Twinrix) is used, give 3 doses at 0, 1, and 6 months; alternatively, a 4-dose Twinrix schedule, administered on days 0, 7, and 21–30 followed by a booster dose at month 12 may be used.
• Adult patients receiving hemodialysis or with other immunocompromising conditions should receive 1 dose of 40 µg/mL (Recombivax HB) administered on a 3-dose schedule at 0, 1, and 6 months or 2 doses of 20 µg/mL (Engerix-B) administered simultaneously on a 4-dose schedule at 0, 1, 2, and 6 months.
14. Selected conditions for which Haemophilus influenzae type b (Hib) vaccine may be used
• 1 dose of Hib vaccine should be considered for persons who have sickle cell disease, leukemia, or HIV infection, or who have anatomic or functional asplenia if they have not previously received Hib vaccine.
15. Immunocompromising conditions
• Inactivated vaccines generally are acceptable (e.g., pneumococcal, meningococcal, and influenza [inactivated influenza vaccine]), and live vaccines generally are avoided in persons with immune deficiencies or immunocompromising conditions. Information on specific conditions is available at http://www.cdc.gov/vaccines/pubs/acip-list.htm.
|
TABLE. Contraindications and precautions to commonly used vaccines in adults1*† |
||
|---|---|---|
|
Vaccine |
Contraindications |
Precautions |
|
Influenza, inactivated vaccine (IIV) |
Severe allergic reaction (e.g., anaphylaxis) after previous dose of any influenza vaccine or to a vaccine component, including egg protein. |
Moderate or severe acute illness with or without fever. History of Guillain-Barré Syndrome (GBS) within 6 weeks of previous influenza vaccination. Persons who experience only hives with exposure to eggs should receive IIV with additional safety precautions.2 |
|
Influenza, live attenuated (LAIV)3 |
Severe allergic reaction (e.g., anaphylaxis) after previous dose of any influenza vaccine or to a vaccine component, including egg protein. Conditions for which the Advisory Committee on Immunization Practices (ACIP) recommends against use, but which are not contraindications in vaccine package insert: immune suppression, certain chronic medical conditions such as asthma, diabetes, heart or kidney disease. and pregnancy.4 |
Moderate or severe acute illness with or without fever. History of GBS within 6 weeks of previous influenza vaccination. Receipt of specific antivirals (i.e., amantadine, rimantadine, zanamivir, or oseltamivir) 48 hours before vaccination. Avoid use of these antiviral drugs for 14 days after vaccination. |
|
Tetanus, diphtheria, pertussis (Tdap); tetanus, diphtheria (Td) |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component. For pertussis-containing vaccines: encephalopathy (e.g., coma, decreased level of consciousness, or prolonged seizures) not attributable to another identifiable cause within 7 days of administration of a previous dose of Tdap or diphtheria and tetanus toxoids and pertussis (DTP) or diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine. |
Moderate or severe acute illness with or without fever. GBS within 6 weeks after a previous dose of tetanus toxoid–containing vaccine. History of arthus-type hypersensitivity reactions after a previous dose of tetanus or diptheria toxoid–containing vaccine; defer vaccination until at least 10 years have elapsed since the last tetanus toxoid-containing vaccine. For pertussis-containing vaccines: progressive or unstable neurologic disorder, uncontrolled seizures, or progressive encephalopathy until a treatment regimen has been established and the condition has stabilized. |
|
Varicella2 |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component. Known severe immunodeficiency (e.g., from hematologic and solid tumors, receipt of chemotherapy, congenital immunodeficiency, or long-term immunosuppressive therapy5 or patients with human immunodeficiency virus (HIV) infection who are severely immunocompromised). Pregnancy. |
Recent (within 11 months) receipt of antibody-containing blood product (specific interval depends on product).6,7 Moderate or severe acute illness with or without fever. Receipt of specific antivirals (i.e., acyclovir, famciclovir, or valacyclovir) 24 hours before vaccination; avoid use of these antiviral drugs for 14 days after vaccination. |
|
Human papillomavirus (HPV) |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component. |
Moderate or severe acute illness with or without fever. Pregnancy. |
|
Zoster |
Severe allergic reaction (e.g., anaphylaxis) to a vaccine component. Known severe immunodeficiency (e.g., from hematologic and solid tumors, receipt of chemotherapy, or long-term immunosuppressive therapy5 or patients with HIV infection who are severely immunocompromised). Pregnancy. |
Moderate or severe acute illness with or without fever. Receipt of specific antivirals (i.e., acyclovir, famciclovir, or valacyclovir) 24 hours before vaccination; avoid use of these antiviral drugs for 14 days after vaccination. |
|
Measles, mumps, rubella (MMR)3 |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component. Known severe immunodeficiency (e.g., from hematologic and solid tumors, receipt of chemotherapy, congenital immunodeficiency, or long-term immunosuppressive therapy5 or patients with HIV infection who are severely immunocompromised). Pregnancy. |
Moderate or severe acute illness with or without fever. Recent (within 11 months) receipt of antibody-containing blood product (specific interval depends on product).6,7 History of thrombocytopenia or thrombocytopenic purpura. Need for tuberculin skin testing.8 |
|
TABLE. (Continued) Contraindications and precautions to commonly used vaccines in adults1*† |
||
|
Vaccine |
Contraindications |
Precautions |
|
Pneumococcal polysaccharide (PPSV) |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component. |
Moderate or severe acute illness with or without fever. |
|
Pneumococcal conjugate (PCV13) |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component, including to any vaccine containing diphtheria toxoid. |
Moderate or severe acute illness with or without fever. |
|
Meningococcal, conjugate, (MCV4); meningococcal, polysaccharide (MPSV4) |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component. |
Moderate or severe acute illness with or without fever. |
|
Hepatitis A (HepA) |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component. |
Moderate or severe acute illness with or without fever. |
|
Hepatitis B (HepB) |
Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component. |
Moderate or severe acute illness with or without fever. |
|
1. Vaccine package inserts and the full ACIP recommendations for these vaccines should be consulted for additional information on vaccine-related contraindications and precautions and for more information on vaccine excipients. Events or conditions listed as precautions should be reviewed carefully. Benefits of and risks for administering a specific vaccine to a person under these circumstances should be considered. If the risk from the vaccine is believed to outweigh the benefit, the vaccine should not be administered. If the benefit of vaccination is believed to outweigh the risk, the vaccine should be administered. A contraindication is a condition in a recipient that increases the chance of a serious adverse reaction. Therefore, a vaccine should not be administered when a contraindication is present. 2. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 influenza season. MMWR 2012;61:613-8. 3. LAIV, MMR, and varicella vaccines can be administered on the same day. If not administered on the same day, these live vaccines should be separated by at least 28 days. 4. For a complete list of conditions that CDC considers to be reasons to avoid getting LAIV, see CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59(No. RR-8). Available at http://www.cdc.gov/vaccines/pubs/acip-list.htm. 5. Immunosuppressive steroid dose is considered to be 2 or more weeks of daily receipt of 20 mg prednisone or the equivalent. Vaccination should be deferred for at least 1 month after discontinuation of such therapy. Providers should consult ACIP recommendations for complete information on the use of specific live vaccines among persons on immune-suppressing medications or with immune suppression because of other reasons. 6. Vaccine should be deferred for the appropriate interval if replacement immune globulin products are being administered. 7. See CDC. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. RR-2). Available at http://www.cdc.gov/vaccines/pubs/acip-list.htm. 8. Measles vaccination might suppress tuberculin reactivity temporarily. Measles-containing vaccine may be administered on the same day as tuberculin skin testing. If testing cannot be performed until after the day of MMR vaccination, the test should be postponed for at least 4 weeks after the vaccination. If an urgent need exists to skin test, do so with the understanding that reactivity might be reduced by the vaccine. |
||
|
* Adapted from CDC. Table 6. Contraindications and precautions to commonly used vaccines. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR 2011;60(No. RR-2):40–41 and from Atkinson W, Wolfe S, Hamborsky J, eds. Appendix A. Epidemiology and prevention of vaccine preventable diseases. 12th ed. Washington, DC: Public Health Foundation, 2011. Available at http://www.cdc.gov/vaccines/pubs/pinkbook/index.html. † Regarding latex allergy. Consult the package insert for any vaccine administered. |
||
See footnotes on page 18.
Advisory Committee on Immunization Practices
Membership List, October 2012
Chair: Jonathan Temte, MD, PhD, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin
Executive Secretary: Larry Pickering, MD, National Center for Immunization and Respiratory Diseases, CDC, Atlanta, Georgia.
Members: Nancy Bennett, MD, University of Rochester School of Medicine and Dentistry, Rochester, New York; Joseph Bocchini, Jr., MD, Louisiana State University Health Sciences Center, Shreveport, Louisiana; Douglas Campos-Outcalt, MD, University of Arizona College of Medicine, Phoenix, Arizona; Tamera Coyne-Beasley, MD, University of North Carolina, Chapel Hill, North Carolina; Jeffrey Duchin, MD, University of Washington, Seattle, Washington; Kathleen Harriman, PhD, California Department of Public Health, Richmond, CA; Lee Harrison, MD, University of Pittsburgh, Pittsburgh, Pennsylvania; Renée Jenkins, MD, Howard University School of Medicine, District of Columbia; Ruth Karron, MD, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland; Wendy Keitel, MD, Baylor College of Medicine, Houston, Texas; Sara Rosenbaum, JD, Georgetown University, District of Columbia; Lorry Rubin, MD, Steven and Alexandra Cohen Children's Medical Center of New York, New Hyde Park, New York; Mark Sawyer, MD, University of California at San Diego, California; Marietta Vázquez, MD, Yale University School of Medicine, New Haven, Connecticut.
Ex Officio Members: Vito Caserta, MD, MPH, Health Resources and Services Administration, Rockville, Maryland; Jesse Geibe, MD, Department of Defense, CDC, Atlanta, Georgia; Bruce Gellin, MD, National Vaccine Program Office, District of Columba; Richard Gorman, MD, National Institutes of Health, Bethesda, Maryland; Amy Groom, MPH, Indian Health Service, Albuquerque, New Mexico; Mary Beth Hance, Centers for Medicare and Medicaid Services, Baltimore, Maryland; Linda Kinsinger, MD, Department of Veterans Affairs, Durham, North Carolina; Wellington Sun, MD, Food and Drug Administration, Bethesda, Maryland.
Liaison Representatives: American Academy of Family Physicians, Jamie Loehr, MD, Cayuga Family Medicine, Ithaca, New York; American Academy of Pediatrics, Michael Brady, MD, Ohio State University, Columbus, Ohio, David Kimberlin, MD, University of Alabama School of Medicine, Birmingham, Alabama; American Academy of Physician Assistants, Marie-Michèle Léger, MPH, Alexandria, Virginia; American College Health Association, James C. Turner, MD, University of Virginia School of Medicine, Charlottesville, Virginia; American College of Obstetricians and Gynecologists, Laura Riley, MD, Harvard Medical School, Boston, Massachusetts; American College of Physicians, Gregory Poland, MD, Mayo Clinic, Rochester, Minnesota; American College of Physicians, Sandra Fryhofer, MD, Emory University School of Medicine, Atlanta, Georgia; American Geriatrics Society, Kenneth Schmader, MD, Duke University and Durham VA Medical Centers, Durham, North Carolina; America›s Health Insurance Plans, Mark Netoskie, MD, CIGNA, Houston, Texas; American Medical Association, Litjen Tan, PhD, Chicago, Illinois; American Nurses Association, Katie Brewer, MSN, Silver Springs, Maryland; American Osteopathic Association, Stanley Grogg, DO, Oklahoma State University-Center for Health Sciences, Tulsa, Oklahoma; American Pharmacists Association, Stephan L. Foster, PharmD, University of Tennessee Health Sciences Center, College of Pharmacy, Memphis, Tennessee; Association of Immunization Managers, Kelly Moore, MD, Tennessee Department of Health, Nashville, Tennessee; Association for Prevention Teaching and Research, W. Paul McKinney, MD, University of Louisville School of Public Health and Information Sciences, Louisville, Kentucky; Association of State and Territorial Health Officials, José Montero, MD, New Hampshire Department of Health and Human Services, Concord, New Hampshire; Biotechnology Industry Organization, Clement Lewin, PhD, Novartis Vaccines and Diagnostics, Cambridge, Massachusetts; Canadian National Advisory Committee on Immunization, Bryna Warshawsky, MDCM, Middlesex-London Health Unit, London, Ontario, Canada; Council of State and Territorial Epidemiologists, Christine Hahn, MD, Idaho Department of Health and Welfare, Boise, Idaho; Department of Health, United Kingdom, David M. Salisbury, MD, London, United Kingdom; Healthcare Infection Control Practices Advisory Committee, Alexis Elward, MD, Washington University School of Medicine, St Louis, Missouri; Infectious Diseases Society of America, Kathleen Neuzil, MD, University of Washington School of Medicine, Seattle, Washington; Infectious Diseases Society of America, Carol Baker, MD, Baylor College of Medicine, Houston, Texas; National Association of County and City Health Officials, Matthew Zahn, MD, Orange County Health Care Agency, Santa Ana, California; National Association of Pediatric Nurse Practitioners, Patricia Stinchfield, MPH, Children's Hospitals and Clinics of Minnesota, St Paul, Minnesota; National Foundation for Infectious Diseases, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tennessee; National Medical Association, Patricia Whitley-Williams, MD, University of Medicine and Dentistry of New Jersey, New Brunswick, New Jersey; National Vaccine Advisory Committee, Walter Orenstein, MD, Emory University School of Medicine, Atlanta, Georgia; Pharmaceutical Research and Manufacturers of America, Damian A. Braga, Swiftwater, Pennsylvania; Society for Adolescent Health and Medicine, Amy Middleman, MD, Baylor College of Medicine, Houston, Texas; Society for Healthcare Epidemiology of America, Harry Keyserling, MD, Emory University School of Medicine, Atlanta, Georgia.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


