Interim Results: Influenza A (H1N1) 2009 Monovalent Vaccination Coverage --- United States, October--December 2009
Early Release
January 15, 2010 / 59(Early Release);1-5
In July 2009, the Advisory Committee on Immunization Practices (ACIP) issued recommendations for use of the influenza A (H1N1) 2009 monovalent vaccine (1). Recognizing that the vaccine supply would not be ample immediately but would grow over time, ACIP identified 1) initial target groups, consisting of approximately 160 million persons, and 2) a limited vaccine subset of the target groups, initially estimated at 42 million persons (and more recently estimated at 62 million persons), to receive first priority while the 2009 H1N1 vaccine supply was limited (1). ACIP recommended expanding vaccination to the rest of the population as vaccine supplies increased. To estimate 2009 H1N1 vaccination coverage to date for the 2009--10 influenza season, CDC analyzed results from the National 2009 H1N1 Flu Survey (NHFS) and the Behavioral Risk Factor Surveillance System (BRFSS) survey, conducted during December 27, 2009--January 2, 2010, and December 1--27, 2009, respectively. The results indicated that, as of January 2, an estimated 20.3% of the U.S. population (61 million persons) had been vaccinated, including 27.9% of persons in the initial target groups and 37.5% of those in the limited vaccine subset. An estimated 29.4% of U.S. children aged 6 months--18 years had been vaccinated. Now that an ample supply of 2009 H1N1 vaccine is available, efforts should continue to increase vaccination coverage among persons in the initial target groups and to offer vaccination to the rest of the U.S. population, including those aged ≥65 years (2).
To provide both timely estimates of 2009 H1N1 vaccination coverage and reliable estimates of coverage in priority populations (e.g., the initial target groups and the limited vaccine subset*), CDC used two separate surveys, NHFS and BRFSS. NHFS is a new survey, scheduled to operate from October 2009 through June 2010 to track 2009 H1N1 and seasonal influenza vaccination coverage nationally on a weekly basis. NHFS is a random-digit--dialed telephone survey based on a rolling weekly sample of respondents with landline and cellular telephones. Monthly targets were set to achieve approximately 4,889 completed interviews from landline households and 1,111 from cellular-only or cellular-mostly households, or approximately 6,000 interviews in all. To determine influenza vaccination status, respondents were asked whether they (or their child) had received "an H1N1 flu vaccination" since September, and if so, in which month.† The NHFS estimates presented in this report show the percentage of respondents interviewed during the week of December 27, 2009--January 2, 2010, who reported receiving vaccine from October 1, 2009 to the date of interview. Unvaccinated NHFS respondents also were asked: "How likely are you to get an H1N1 flu vaccination between now and June 2010?"
Because the weekly sample sizes from NHFS are not large enough for reliable estimation of vaccination coverage among persons in individual initial target groups, CDC also used BRFSS, which collected vaccination coverage data for most of the initial target groups on a monthly basis. BRFSS conducts state-based, random-digit--dialed telephone surveys of the noninstitutionalized U.S. population aged ≥18 years to determine the prevalence of health conditions and health risk behaviors. Since 2001, BRFSS has included questions on seasonal influenza vaccination in its core survey. To determine 2009 H1N1 vaccination coverage, BRFSS respondents in 49 states (all except Vermont) and the District of Columbia were asked if they (or their child in 39 of these states and the District of Columbia) had been vaccinated for the "H1N1 flu" since September, and if so, in which month?§ BRFSS results in this report represent the percentage of respondents who reported receiving 2009 H1N1 vaccine during the period from October 1, 2009, through the date of interview during December 1--27, 2009.
For both NHFS and BRFSS, respondents with missing influenza vaccination information were excluded. Results from both surveys were weighted to reflect selected demographic and geographic population estimates and analyzed by statistical software that accounts for survey design. Statistical significance of differences was assessed by t-test. For NHFS, the Council of American Survey and Research Organizations (CASRO) response rate for the first 13 weekly sample groups was 34% for landline telephone respondents and 26% for cellular telephone respondents; the cooperation rate was 43% for landline and 57% for cellular. During December 2009, the BRFSS median CASRO response and cooperation rates were 50% and 74%, respectively.¶
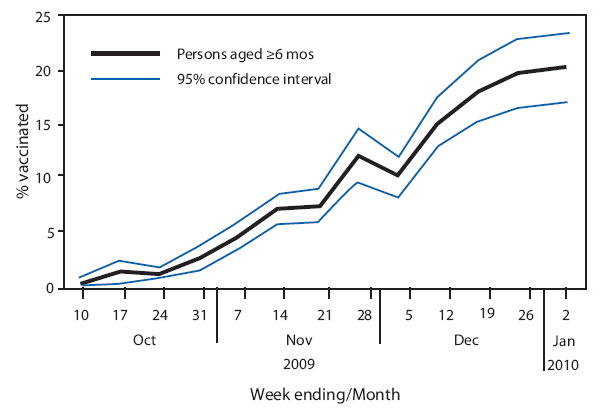
From October 10, 2009 to January 2, 2010, the weekly NHFS percentage of U.S. residents who reported they had received at least 1 dose of 2009 H1N1 vaccine rose to 20.3% (Figure). According to NHFS data, of the 24 million vaccine doses administered in the United States through mid-November, an estimated 21 million (85%) went to persons in the initial 2009 H1N1 target groups. By the end of December, this percentage had declined to 74% (48 million of the 65 million doses administered). For the survey week December 27, 2009--January 2, 2010, NHFS data indicated that 29.4% of children aged 6 months--18 years (22 million) had received at least 1 dose of vaccine, including 33.0% of children aged 6 months--4 years (Table 1). Among children aged 6 months--9 years, an age group recommended to receive 2 doses of 2009 H1N1 vaccine, 34.6% (95% confidence interval [CI] = 26.6%-- 42.6%) had received at least 1 dose; among these children, 17.8% (CI = 10.1%-- 25.5%) had received 2 doses.
According to NHFS estimates, vaccination coverage was 27.9% among persons included in the 2009 H1N1 initial target groups and 37.5% among those in the limited vaccine subset, two populations estimated to number 160 million (CI = 144--176 million) and 62 million (CI = 51--73 million) respectively in the United States (Table 1). Among BRFSS survey respondents during December 1--27, estimated coverage for specific initial target groups was 38.0% for pregnant women, 22.3% for health-care personnel, and 11.6% for adults aged 25--64 years with high-risk medical conditions. Among NHFS respondents during November 29--December 26, coverage was 13.9% for adults who live with or provide care for infants aged <6 months (Table 2).
BRFSS estimates of 2009 H1N1 vaccination rates generally were higher among non-Hispanic whites than among non-Hispanic blacks. However, this difference was statistically significant only among adults aged 25--64 years with high-risk conditions (13.1% [CI = 11.1%--15.1%] versus 5.4% [CI = 2.5%--8.3%]) and health-care personnel (25.6% [CI = 22.5%--28.7%] versus 7.6% [CI = 3.3%--11.9%]).
Among the December 27--January 2 NHFS participants who had not yet received 2009 H1N1 vaccination, 10.9% (CI = 7.4%--14.4%) said they definitely intended to get vaccinated by June 2010; an additional 22.5% (CI = 18.6%--26.4%) said they would probably get vaccinated. Among parents of unvaccinated children, 21.1% (CI = 10.7%-- 31.5%) said they definitely intended to have their children vaccinated, and 17.7% (CI = 10.6%--24.8%) said they probably would have their children vaccinated.
Reported by
JA Singleton, MS, TA Santibanez, PhD, PJ Lu, PhD, H Ding, MD, GL Euler, DrPH, Immunization Svc Div, GL Armstrong, MD, Div of Viral Diseases, BP Bell, MD, National Center for Immunization and Respiratory Diseases; M Town, MS, L Balluz, ScD, Div of Adult and Community Health, National Center for Chronic Disease Prevention and Health Promotion, CDC.
Editorial Note
Development of 2009 H1N1 vaccines began immediately after the virus emerged in late April 2009. By late June, several manufacturers had begun the process of producing vaccines; within 4 months, vaccines had been licensed by the Food and Drug Administration, and the first lots of vaccine were released for use in the United States. By mid-December, approximately 85 million doses had been shipped to providers around the country. During October 5--December 31, a period of limited vaccine supply, vaccination efforts focused on those groups at highest risk for influenza or influenza complications or persons in close contact with those at high risk (1). This report indicates that, by the beginning of 2010, an estimated 20% of the population, or 61 million U.S. residents, had received 2009 H1N1 vaccine. Of persons in the groups initially targeted by ACIP for vaccination, an estimated 28% reported receiving 2009 H1N1 vaccine. The highest coverage (approximately 38%) was achieved among persons in the limited vaccine subset, as defined by ACIP, indicating that public health efforts largely were effective at directing available vaccine to those persons who needed it most.
Overall, the 29% 2009 H1N1 vaccination coverage among children aged 6 months--18 years was similar to estimates of seasonal influenza vaccination coverage (24%--27%) for this age group during the 2008--09 influenza season (3,4). Among children aged <5 years, who have been recommended for seasonal influenza vaccination since 2006 (5) and who have been among the groups most severely affected by 2009 H1N1, first-dose 2009 H1N1 influenza vaccination coverage was 33%, approaching seasonal influenza vaccination coverage estimates (35%--43%) during recent seasons (3,4).
Hospitalization rates and mortality from 2009 H1N1 influenza have been high among pregnant women (6,7). The 38% 2009 H1N1 vaccination coverage among pregnant women in this report was higher than the rate typically achieved (15%--25%) for seasonal influenza vaccination (8). However, the CI around this estimate is large (24%--52%). A separate system, the Pregnancy Risk Assessment Monitoring System (PRAMS), is collecting data, including influenza immunization coverage, from approximately 30,000 women with live births in 31 states and will provide more precise estimates in the future. To improve influenza vaccination coverage among pregnant women this year and during future seasons, efforts should continue to urge obstetricians and other health-care providers to provide influenza vaccine to pregnant women.
The results in this report show that nearly 90% of adults aged <65 years with medical conditions that increase their risk for influenza-related complications remain unvaccinated. Among adults hospitalized with 2009 H1N1 infection, approximately three fourths had at least one high-risk condition (e.g., asthma, chronic obstructive pulmonary disease, diabetes and chronic cardiovascular disease) (9). Given the increased supply of vaccine, efforts to encourage 2009 H1N1 vaccination among persons at increased risk for 2009 H1N1 influenza complications should be strengthened.
Seasonal influenza vaccination coverage among health-care workers historically has been below 50% (8). Efforts to vaccinate health-care workers began when 2009 H1N1 vaccine first became available, but according to the BRFSS survey, during December 1--27, only 22% of health-care workers reported having been vaccinated. Unvaccinated health-care workers who become infected risk transmitting the virus to their family members or patients, who often are at high risk for severe influenza. The current high percentage of unvaccinated health-care workers highlights the need to strengthen measures to improve their influenza vaccination coverage.
Among adults with chronic medical conditions, NHFS and BRFSS show lower vaccination coverage among blacks than whites. Similar disparities have been identified for seasonal influenza and pneumococcal polysaccharide vaccination (4). The finding of lower 2009 H1N1 vaccination coverage among black health-care workers suggests that access to care is not the only barrier to influenza vaccination and highlights a role for targeted outreach efforts.
The findings in this report are subject to at least three limitations. First, the NHFS results presented in this analysis are based on data collected during a single week of interviews, and all results are based on self-report or parental report of 2009 H1N1 vaccination. Because of the limited size of the NHFS sample, confidence limits around estimates are large and final estimates might differ. Second, BRFSS and NHFS are subject to selection bias because of noninclusion of households with only cellular telephones (BRFSS) and households with no telephone service (BRFSS and NHFS). Finally, CASRO response rates and cooperation rates were low, particularly for NHFS.
Although influenza activity has declined in the United States in recent weeks, cases of 2009 H1N1 influenza, including cases of severe disease, continue to occur. The epidemiology of 2009 H1N1 influenza over the months ahead is unknown, but another rise in incidence, as occurred during the winter of the 1957--58 pandemic, remains possible (10). In addition, increases in influenza activity from seasonal influenza also might occur as the season progresses. Vaccination remains the best way to prevent influenza infection and influenza-related hospitalizations and deaths.
Acknowledgments
The findings in this report are based, in part, on NHFS contributions by M Montgomery, K Copeland, N Davis, and others at the National Opinion Research Center, Chicago, Illinois; data collected by state BRFSS coordinators; members of the CDC H1N1 Vaccine Coverage Monitoring Team; and members of the CDC Behavioral Surveillance Branch, Atlanta, GA.
References
- CDC. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-10).
- CDC. National Influenza Vaccination Week---January 10--16, 2010. MMWR 2010;58(51&52);1444.
- CDC. Influenza vaccination coverage among children and adults---United States, 2008--09 season. MMWR 2009;58:1091--5.
- CDC. Vaccination coverage estimates from the National Health Interview Survey: United States, 2008. Updated July 22, 2009. Available at http://www.cdc.gov/nchs/data/hestat/vaccine_coverage.htm. Accessed January 14, 2009.
- Finelli L, Fiore A, Dhara R, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 2008;122:805--11.
- Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374:451--8.
- Louie JK, Acosta M, Jamieson DJ, Honein MA, California Pandemic (H1N1 Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010;362:27--35.
- CDC. Prevention and control of seasonal influenza with vaccines---recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-8):26.
- Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April--June 2009. N Engl J Med 2009;361:1935--44.
- Henderson DA, Courtney B, Inglesby TV, et al. Public health and medical responses to the 1957--58 influenza pandemic. Biosecur Bioterror 2009;7:265--73.
|
What is already known on this topic?
Since 2009 H1N1 influenza vaccine first became available in October 2009, public health agencies have directed limited vaccine supplies toward groups of persons who can most benefit from the vaccine.
What is added by this report?
By the end of December 2009, an estimated 61 million persons (20% of the U.S. population) had been vaccinated, including 27.9% of persons in the initial target groups, 29.4% of children, 11.6% of adults aged 25--64 years with underlying medical conditions, 22.3% of health-care personnel, and 13.9% of adults caring for infants aged <6 months.
What are the implications for public health practice?
Now that there is ample supply of vaccine, efforts should continue to improve vaccination coverage among persons in initial target groups, as well as to offer vaccination to the rest of the U.S. population, including those aged ≥65 years.
|
FIGURE. Weekly estimates of influenza A (H1N1) 2009 monovalent vaccination coverage among U.S. residents aged ≥6 months --- National 2009 H1N1 Flu Survey, week ending October 10, 2009, through week ending January 2, 2010
Alternative Text: The figure above shows weekly estimates of influenza A (H1N1) 2009 monovalent vaccination coverage among U.S. residents aged ≥6 months for the week ending October 10, 2009, through the week ending January 2, 2010. During that period, the percentage of persons reporting receipt of 2009 H1N1 vaccination increased to 20.3%
|
Age group/Priority group
|
U.S. population (millions)
|
H1N1 vaccination coverage
|
|
No. surveyed†
|
% vaccinated (95% CI§)
|
Estimated no. of persons vaccinated (millions) (95% CI)
|
|
Age group
|
|
|
|
|
|
|
|
Total ≥6 mos
|
299
|
3,023
|
20.3
|
(17.2--23.4)
|
61
|
(51--70)
|
|
6 mos--4 yrs
|
19
|
500
|
33.0
|
(21.6--44.4)¶
|
6
|
(4--8)
|
|
6 mos--18 yrs
|
76
|
1,638
|
29.4
|
(23.8--35.0)
|
22
|
(18--27)
|
|
6 mos--24 yrs
|
101
|
1,716
|
25.9
|
(20.6--31.2)
|
26
|
(21--32)
|
|
6 mos--64 yrs
|
261
|
2,672
|
21.7
|
(18.3--25.1)
|
57
|
(48--66)
|
|
5--18 yrs
|
57
|
1,138
|
28.1
|
(21.7--34.5)
|
16
|
(12--20)
|
|
≥19 yrs
|
223
|
1,385
|
17.3
|
(13.8--20.8)
|
39
|
(31--46)
|
|
19--64 yrs
|
185
|
1,034
|
18.6
|
(14.5--22.7)
|
34
|
(27--42)
|
|
≥65 years
|
38
|
351
|
11.2
|
(6.5--15.9)
|
4
|
(2--6)
|
|
Priority group
|
|
|
|
|
|
|
|
Initial target groups**
|
160
|
2,101
|
27.9
|
(23.5--32.3)
|
45
|
(38--52)
|
|
Limited vaccine subset††
|
62
|
807
|
37.5
|
(30.1--44.9)
|
23
|
(19--28)
|
|
Initial target group
|
H1N1 vaccination coverage
|
|
No. surveyed§
|
% vaccinated (95% CI¶)
|
|
Adults aged 25--64 years with high-risk conditions**
|
4,044
|
11.6
|
(9.9--13.3)
|
|
Health-care personnel††
|
3,329
|
22.3
|
(19.6--25.0)
|
|
Pregnant women
|
150
|
38.0
|
(24.3--51.7)§§
|
|
Adults living or caring for infant aged <6 months (NHFS¶¶)
|
402
|
13.9
|
(9.2--18.6)
|
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication. |
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
![]() .
.
 Twitter
Twitter Facebook
Facebook LinkedIn
LinkedIn Email
Email Digg
Digg Add this to your site
Add this to your site