FIGURE 1. Laboratory-confirmed wild poliovirus type 1 cases (N = 458), by week of paralysis onset and age group --- Tajikistan, 2010
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Outbreaks Following Wild Poliovirus Importations --- Europe, Africa, and Asia, January 2009--September 2010
Weekly
November 5, 2010 / 59(43);1393-1399The Global Polio Eradication Initiative (GPEI) began in 1988. By 2006, indigenous transmission of wild poliovirus (WPV) had been interrupted in all but four countries (Afghanistan, India, Nigeria, and Pakistan) (1). However, outbreaks following WPV importations into previously polio-free countries remain an ongoing risk until polio is eradicated (1--3). The GPEI Strategic Plan for 2010--2012 (4) set the following two goals for outbreak control: 1) end outbreaks occurring in 2009 by mid-2010 and 2) end outbreaks occurring during 2010 to mid-2012 within 6 months of confirmation. This report describes new outbreaks that have occurred in the World Health Organization (WHO) European Region and updates previous reports on the status of outbreaks in Africa and Asia (3). In 2010, the first WPV importation into the European Region since the region was declared polio-free in 2002 resulted in 476 confirmed cases: 458 in Tajikistan, 14 in Russia, three in Turkmenistan, and one in Kazakhstan. In Africa and Asia, 11 new importations into six countries were observed in 2010; 30 WPV importations that occurred during 2008--2009 resulted in 215 cases in 15 African countries during 2009--2010. An outbreak is considered interrupted if 6 months have elapsed since the latest confirmed case and surveillance performance indicators meet WHO standards. All 2009 outbreaks in Africa appear to have been interrupted, and 2010 outbreaks in three countries appear to have been interrupted. Maintaining high routine vaccination coverage and sensitive surveillance at all times and rapidly instituting additional immunization programs to control outbreaks are key to limiting and stopping the spread of WPV.
European Region Importations, 2010
On April 13, 2010, Tajikistan notified WHO of a sharp increase in reported acute flaccid paralysis (AFP) cases. On April 20, the designated WHO regional reference laboratory in Moscow, Russia, identified WPV type 1 (WPV1) in stool specimens from persons with AFP cases; the WPV1 was genetically most closely related to WPV1 isolated in Uttar Pradesh, India, in August 2009. As of November 1, 2010, Tajikistan had reported 458 laboratory-confirmed WPV1 cases from 35 of 61 administrative territories, with paralysis onset dates occuring February 1--July 4 (Figure 1). Ninety (20%) patients were aged <1 year, 208 (45%) were aged 1--4 years, 107 (23%) were aged 5--14 years, and 53 (12%) were aged ≥15 years. Early in the outbreak, the majority of cases were in children aged <5 years; after week 20, 78% of cases were in persons aged ≥5 years (Figure 1). Twenty-six (5.7%) patients died; 15 were aged <5 years, eight were aged 5--14 years, and three were aged ≥15 years.
The outbreak spread to three other polio-free countries,* where 18 cases were confirmed, bringing to 476 the total number of cases in the European Region (Figure 2). Russia reported 14 cases following at least five independent importations, with onsets during May 4--September 25 (confirmed May 31). Turkmenistan reported three cases in June (confirmed June 27), and Kazakhstan reported one case in August (confirmed October 5) (Table 1). Nine of these 18 patients were aged <5 years.
Efforts to control the outbreak in Tajikistan began May 4 with the first of four rounds of national supplementary immunization activities (SIAs),† 2 weeks apart, using monovalent type 1 oral poliovirus vaccine (mOPV1) (Table 2). The first two SIAs targeted children aged <6 years, and the third and fourth SIAs targeted children aged <15 years. Mop-up SIAs with mOPV1 were conducted in 34 districts in September; one national SIA using trivalent OPV was conducted in early October, and another is planned for November, each targeting children aged <15 years (Table 2). Reported vaccination coverage for each SIA was ≥98% of the target (Table 2).
In early May 2010, the risk for importation was assessed for 12 countries§ in the European Region to identify high-risk subnational areas for WPV transmission. National authorities were advised to strengthen surveillance¶ through enhanced active case finding and weekly reporting and to implement SIAs as needed. Kazakhstan, Kyrgyzstan, Turkmenistan, and Uzbekistan conducted national SIAs to limit further spread; Russia conducted focal mop-up SIAs and catch-up immunizations (Table 2).
Importations in Africa and Asia, 2009--2010
Nineteen importations of WPV1 and 11 of WPV type 3 (WPV3) that occurred during 2008--2009 (nine in 2008 and 21 in 2009) resulted in 208 polio cases in 15 countries in 2009 and seven additional cases in 2010 (Table 1). As of November 1, 2010, no other 2009 outbreak-related cases had been detected during the 6 months since the latest case in Mauritania (April 28, 2010). In outbreaks with the first case occurring in 2010, seven importations of WPV1 and two of WPV3 resulted in 26 polio cases in four African countries, and WPV1 importation into one Asian country resulted in six cases to date (Table 1).
West Central Africa.** In 2009, outbreaks related to increased circulation of WPV1 and WPV3 in Nigeria during 2008--2009 occurred in 12 countries†† (Table 1). Outbreaks in Mali, Mauritania, and Sierra Leone continued into 2010.
In 2010, Senegal had three importations (first confirmed January 18). New importations with no or limited subsequent transmission also occurred in Liberia (confirmed April 14), Mali (WPV1 confirmed April 8 and WPV3 confirmed October 15) and Niger (confirmed April 22) (Table 1). The most recent case among 2010 outbreaks occurred on September 8 in Liberia.
Horn of Africa. Outbreaks occurred in Kenya and Uganda in 2009 (the latest cases on July 30 and May 10, 2009, respectively) (Table 1). These represented two distinct importations from south Sudan, where WPV1 genetically related to viruses from the importation-related outbreak during 2004--2005 in Sudan was again confirmed from polio cases during June 2008--June 2009.§§ A new 2010 importation case occurred in Uganda on September 28 (confirmed October 18), genetically related to virus last isolated in Kenya in 2009.
South Central Africa. Two cases occurred in Burundi in 2009, most recently on September 12, 2009 (Table 1). This outbreak spread from the Democratic Republic of the Congo (DRC) as a result of WPV1 importation from India into Angola in 2005 and subsequently into DRC (3).
Nepal. Two WPV1 importations from India caused six confirmed WPV1 cases in Nepal in 2010. The first case occurred on February 19 (confirmed on March 19), and the most recent occurred on August 30.
Reported by
Vaccine Preventable Diseases and Immunization, World Health Organization Regional Office for Europe, Copenhagen, Denmark; Polio Eradication Dept, World Health Organization, Geneva, Switzerland. Div of Viral Diseases, Global Immunization Div, National Center for Immunization and Respiratory Diseases, CDC.
Editorial Note
The large 2010 WPV1 outbreak in the WHO European Region, certified as polio-free since 2002, highlights the risk for WPV reintroduction for all countries posed by international travel and migration. Factors contributing to the scale of the outbreak in Tajikistan included a health system with limited resources, accumulation of susceptible persons in areas of low OPV coverage (5), and delays in recognizing and testing the initial cluster of AFP cases, as also occurred during the 2005 outbreak in Yemen (2). In June 2009, the European Regional Commission for the Certification of Poliomyelitis Eradication highlighted a high risk for transmission in Tajikistan if WPV was introduced (6), but funds were not available to conduct preventive SIAs. Additional SIAs are planned in the Central Asian republics and Russia to end the outbreaks and prevent spread to other countries in the region known to have pockets of low vaccination coverage (e.g., Bulgaria, Georgia, and Ukraine). All countries in the region must ensure full political commitment to undertake the actions recommended by WHO to detect WPV importations and limit spread.
The GPEI Strategic Plan milestone of ending 2009 importation-related outbreaks by mid-2010 appears to have been met, with one possible exception: WPV1 circulation related to the 2009 outbreak in Kenya, as suggested by the September 28, 2010, case in Uganda. Whether WPV1 circulating in Kenya in 2009 continued to circulate without detection in Uganda, in Kenya, or in both countries, is uncertain and requires further observation and investigation. Many outbreaks occurring in 2010 have ended or are on track to end within 6 months of confirmation, including the outbreak in Tajikistan. However, concern exists that ongoing transmission within the northern Caucasus area of Russia and in Nepal could spread further, unless high-quality SIAs are implemented. In Africa, some countries that had outbreaks have not met AFP surveillance performance criteria fully, so caution is needed when interpreting the length of time after the latest confirmed cases as a sign that an outbreak has ended, particularly when surveillance is suboptimal in neighboring countries.
During 2009--2010, WPV was imported into polio-free countries from both polio-endemic countries (India and Nigeria) and previously polio-free countries with reestablished transmission (Chad and Sudan) (3), with importations occurring more frequently in countries adjacent to countries with ongoing WPV transmission. The risk for WPV importations in 2010 appears to have decreased as a result of 1) a ≥90% decrease in confirmed cases in Nigeria and northern India compared with the same period in 2009, 2) a prolonged period without confirmed WPV cases in Sudan, and 3) >4 months without confirmed cases in Chad. However, WPV importations from reservoir countries into polio-free areas will continue to occur until transmission is interrupted everywhere.
Transmission after WPV importation can be prevented by ensuring high levels of poliovirus immunity in the population. Early recognition and response to WPV transmission limit the geographic extent and enable more rapid control of an outbreak (8,9). All polio-free countries are advised to maintain high levels of immunity against polioviruses at all times through strong routine vaccination programs, adding SIAs when necessary. Maintaining sensitive, efficient, nationwide AFP surveillance systems with timely investigation and testing of specimens in accredited laboratories is critical to promptly identifying importations. National authorities should maintain updated preparedness plans for timely, large-scale, high-quality response SIAs if WPV importations occur (9).
References
- CDC. Progress toward interruption of wild poliovirus transmission---worldwide, 2009. MMWR 2010;59:545--50.
- CDC. Resurgence of wild poliovirus type 1 transmission and consequences of importation---21 countries, 2002--2005. MMWR 2006;55:145--50.
- CDC. Wild poliovirus type 1 and type 3 importations---15 countries, Africa, 2008--2009. MMWR 2009;58:357--62.
- World Health Organization. Global Polio Eradication Initiative: strategic plan 2010--2012. Geneva, Switzerland: World Health Organization; 2010. Available at http://www.polioeradication.org/content/publications/gpei.strategicplan.2010-2012.eng.may.2010.pdf

 . Accessed October 29, 2010.
. Accessed October 29, 2010. - Tajikistan State Committee for Statistics, UNICEF. Tajikistan living standards measurement survey, 2007. Available at http://www.tojikinfo.tj/en/index.php?news=362
 . Accessed November 1, 2010.
. Accessed November 1, 2010. - World Health Organization Regional Office for Europe. Report of the 22nd meeting of the European Regional Certification Commission for Poliomyelitis Eradication. Copenhagen, Denmark: World Health Organization; 2010. Available at http://www.euro.who.int/__data/assets/pdf_file/0019/92017/E93603.pdf

 . Accessed October 29, 2010.
. Accessed October 29, 2010. - Global Polio Eradication Initiative. Wild poliovirus cases by type, 2009--2010, year to date comparison Geneva, Switzerland: World Health Organization; 2010. Available at http://www.polioeradication.org/casecount.asp
 . Accessed October 29, 2010.
. Accessed October 29, 2010. - Thompson KM, Duintjer Tebbens RJ, Pallansch MA. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal 2006;26:1541--56.
- World Health Organization. Advisory Committee on Polio Eradication: standing recommendations for responding to circulating polioviruses in polio-free areas. Wkly Epidemiol Rec 2005;80:330--1.
* Countries with no evidence of indigenous WPV transmission for ≥1 years and subsequent cases determined to be of external origin by genomic sequencing analysis.
† Mass campaigns conducted for a brief period (days to weeks), during which 1 dose of OPV typically is administered to all children aged <5 years (although the target age group can vary), regardless of vaccination history. Campaigns can be conducted nationally or in portions of the country, and the approach to SIA implementation varies widely by country.
§ Armenia, Azerbaijan, Bosnia and Herzegovina, Georgia, Kyrgyzstan, Kazakhstan, Russia, Tajikistan, Turkey, Turkmenistan, Ukraine, and Uzbekistan.
¶ AFP surveillance quality is monitored by performance indicators that suggest the ease by which any WPV transmission will be detected. The current WHO targets are a nonpolio AFP detection rate of >2 cases per 100,000 population aged <15 years and adequate stool specimen collection from >80% of AFP cases, in which two specimens are collected ≥24 hours apart, both within 14 days of paralysis onset, and shipped on ice or frozen ice packs to a WHO-accredited laboratory, arriving in good condition. National data might mask surveillance system weaknesses at subnational levels.
** Regions are based on GPEI epidemiologic and programmatic considerations and do not necessarily coincide with traditional geographic divisions.
†† Benin, Burkina Faso, Cameroon, Central African Republic, Côte d'Ivoire, Guinea, Liberia, Mali, Mauritania, Niger, Sierra Leone, and Togo.
§§ The latest patient in south Sudan had onset June 27, 2009; however, surveillance quality has not met performance standards for >12 months.
What is already known on this topic?
The four remaining countries that have never interrupted wild poliovirus (WPV) transmission (Afghanistan, India, Nigeria, and Pakistan) and previously polio-free countries with reestablished transmission following WPV importation (Angola, Chad, Democratic Republic of the Congo, and Sudan) continue to be the source of WPV importations into polio-free areas.
What is added by this report?
All 2009 WPV outbreaks (with one possible exception) appear to have been interrupted, but new importations have occurred in 2010, including the first WPV importation into the World Health Organization European Region since the region was certified polio-free in 2002. The European Region importation has resulted in a large-scale outbreak (458 cases) in Tajikistan, with 18 more cases in Kazakhstan, Russia, and Turkmenistan.
What are the implications for public health practice?
All polio-free countries are advised to maintain high vaccination coverage and sensitive surveillance systems. If WPV importation is recognized in a country or its neighbors, health authorities need to institute supplementary immunization activities rapidly to limit WPV spread and interrupt outbreaks.
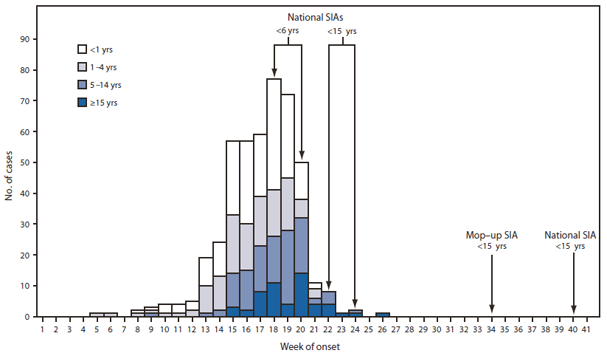
Abbreviation: SIA = supplementary immunization activity.
Alternate Text: The figure above is a histogram showing laboratory-confirmed wild poliovirus type 1 cases (N = 458), by week of paralysis onset and age group in Tajikistan in 2010. As of November 1, 2010, Tajikistan had reported 458 laboratory-confirmed WPV1 cases from 35 of 61 administrative territories, with paralysis onset dates occurring February 1-July 4.
FIGURE 2. Distribution of laboratory-confirmed wild poliovirus type 1 cases (N = 476) --- World Health Organization European Region, 2010
Alternate Text: The figure above shows the distribution of laboratory-confirmed wild poliovirus type 1 cases (N = 476) in four countries in the World Health Organization European Region in 2010: 458 in Tajikistan, 14 in Russia, three in Turkmenistan, and one in Kazakhstan.
|
TABLE 1. Summary information regarding importations of wild poliovirus (WPV) types 1 and 3 into previously polio-free* countries and subsequent outbreaks --- Africa, Europe, and Asia, January 2009--September 2010 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Region/Country |
No. of Importations† by WPV type |
Onset date of first confirmed polio case |
Onset date of most recent polio case |
WPV origin by sequencing |
No. of polio cases confirmed to date§ |
No. of SIAs between first and most recent case¶ |
No. of SIAs since most recent case¶ |
Estimated OPV3 coverage during 2009** |
Surrogate OPV3 coverage†† during 2009 (%) |
|
2009 outbreaks |
|||||||||
|
West Central Africa§§ |
|||||||||
|
Benin |
1 WPV1 |
11/3/2008 |
4/19/2009 |
Nigeria |
20 |
3 |
6 |
83 |
67 |
|
Burkina Faso |
3 WPV1 |
11/4/2008 |
10/25/2009 |
Togo, Côte d'Ivoire, Benin |
15 |
7 |
6 |
84 |
83 |
|
Cameroon |
2 WPV3 |
7/29/2009 |
10/15/2009 |
Nigeria, Chad |
3 |
0 |
6 |
79 |
70 |
|
CAR |
1 WPV3 |
4/2/2009 |
8/3/2009 |
Chad |
14 |
3 |
6 |
47 |
63 |
|
Côte d'Ivoire |
2 WPV1 |
12/24/2008 |
8/6/2009 |
Burkina Faso |
27 |
4 |
5 |
77 |
66 |
|
Guinea |
1 WPV1 |
4/9/2009 |
11/3/2009 |
Côte d'Ivoire |
42 |
4 |
6 |
53 |
66 |
|
Liberia |
1 WPV1 |
4/29/2009 |
10/26/2009 |
Côte d'Ivoire |
11 |
4 |
5 |
74 |
63 |
|
Mali |
2 WPV1 |
8/30/2008 |
03/30/2010 |
Burkina Faso, Guinea |
3§ |
8 |
4 |
74 |
89 |
|
Mauritania |
1 WPV1 |
10/7/2009 |
4/28/2010 |
Cote d'Ivoire |
18§ |
5 |
3 |
63 |
89 |
|
Niger |
8 WPV3 |
12/6/2008 |
5/28/2009 |
Nigeria, Chad |
15 |
2 |
5 |
71 |
87 |
|
1 WPV1 |
5/28/2009 |
--- |
Nigeria |
1 |
--- |
5 |
|||
|
Sierra Leone |
1 WPV1 |
7/15/2009 |
2/28/2010 |
Guinea |
12§ |
5 |
4 |
74 |
75 |
|
Togo |
3 WPV1 |
10/16/2008 |
3/28/2009 |
Burkina Faso, Ghana |
6 |
2 |
4 |
89 |
80 |
|
Horn of Africa |
|||||||||
|
Kenya |
1 WPV1 |
2/3/2009 |
7/30/2009 |
Sudan |
18 |
4 |
5 |
71 |
75 |
|
Uganda |
1 WPV1 |
1/28/2009 |
5/10/2009 |
Sudan |
8 |
4 |
5 |
59 |
76 |
|
South Central Africa |
|||||||||
|
Burundi |
1 WPV1 |
9/8/2009 |
9/12/2009 |
DRC |
2 |
0 |
2 |
96 |
78 |
|
2010 outbreaks |
|||||||||
|
West Central Africa |
|||||||||
|
Liberia |
1 WPV1 |
3/3/2010 |
09/08/2010 |
Guinea |
2 |
4 |
0 |
74 |
63 |
|
Mali |
2 WPV1 |
3/6/2010 |
5/1/2010 |
Mauritania, Burkina Faso |
2 |
2 |
3 |
74 |
89 |
|
1 WPV3 |
09/17/2010 |
--- |
TBD |
1 |
0 |
0 |
|||
|
Niger |
1 WPV3 |
3/8/2010 |
4/1/2010 |
Nigeria |
2 |
0 |
3 |
71 |
87 |
|
Senegal |
3 WPV1 |
1/5/2010 |
4/30/2010 |
Mauritania, Guinea |
18 |
2 |
3 |
83 |
76 |
|
Horn of Africa |
|||||||||
|
Uganda |
1 WPV1 |
09/28/2010 |
--- |
Kenya |
1 |
--- |
0 |
59 |
76 |
|
Europe/Asia |
|||||||||
|
Kazakhstan |
1 WPV1 |
8/12/2010 |
--- |
TBD |
1 |
--- |
1 |
99 |
98 |
|
Nepal |
2 WPV1 |
2/19/2010 |
8/30/2010 |
India |
6 |
6 |
2 |
82 |
97 |
|
Russia |
5 WPV1 |
5/4/2010 |
9/25/2010 |
Tajikistan, TBD |
14 |
0 |
0 |
98 |
89 |
|
Tajikistan |
1 WPV1 |
2/1/2010 |
7/4/2010 |
India |
458 |
4 |
2 |
93 |
100 |
|
Turkmenistan |
2 WPV1 |
6/20/2010 |
6/28/2010 |
Tajikistan |
3 |
0 |
3 |
97 |
100 |
|
Abbreviations: CAR = Central African Republic; DRC = Democratic Republic of the Congo; OPV3 = 3 doses of live, attenuated oral polio virus vaccine; SIAs = supplementary immunization activities; TBD = to be determined. * Countries with no evidence of indigenous WPV transmission for ≥1 years and subsequent cases determined to be of external origin by genomic sequencing analysis. Importations also occurred into Chad and DRC, which in 2009 also experienced reestablished persistent transmission of WPV (≥12 months) after importation. Data as of November 1, 2010. † Detection of one or more polio cases in a country from WPV that genetic analysis showed to be originating from another country. For some outbreaks occurring in 2009, the related importation occuurred in 2008 and transmission continued into 2009: Benin (one importation), Burkina Faso (two), Côte d'Ivoire (one), Mali (one), Niger (three), and Togo (one). Data as of November 1, 2010. § Number of polio cases in a country from WPV importations resulting in outbreaks in that year. For some outbreaks occurring in 2009, additional cases occurred in 2010 that are reflected in the totals for 2009 outbreaks: Mali (one case), Mauritania (five), and Sierra Leone (one). ¶ When ≥25% of children were targeted for vaccination. ** World Health Organization/UNICEF estimate of vaccination coverage with 3 doses of OPV by age 12 months, on the basis of country reports and survey data. Available at http://www.who.int/vaccines/globalsummary/immunization/countryprofileselect.cfm †† Percentage of children aged 6--35 months with nonpolio acute flaccid paralysis (specimen testing does not indicate WPV infection) who have received 3 or more doses of OPV; these national data might mask vaccination coverage weaknesses at subnational levels. §§ Regions are based on Global Polio Eradication Initiative epidemiologic and programmatic considerations and do not necessarily coincide with traditional geographic divisions. |
|||||||||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


