 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Implementation of Newborn Hepatitis B Vaccination --- Worldwide, 2006Globally, hepatitis B virus (HBV) infections are a major cause of cirrhosis and liver cancer and result in an estimated 620,000 deaths annually (1). In 1992, the World Health Organization (WHO) set a goal for all countries to introduce hepatitis B (HepB) vaccine into national routine infant immunization programs by 1997 (2). In countries where a high percentage of HBV infections are acquired perinatally (where general population prevalence of chronic HBV infection is >8%), WHO recommends administering the first HepB vaccine dose <24 hours after birth to prevent perinatal HBV transmission (3). To assess implementation of newborn HepB vaccination, the most recently available data were examined from the Joint Reporting Form used by the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) to track worldwide vaccine coverage for WHO-recommended infant immunizations (4). In 2006, a total of 162 (84%) of 193 countries had introduced HepB vaccine into their national infant immunization schedules. Among the 193 countries, 81 (42%) reported using a schedule with a HepB vaccine birth dose (defined as a dose administered within 24 hours of birth). Worldwide, 27% of newborns received a HepB vaccine birth dose in 2006. In the 87 countries with >8% chronic HBV infection prevalence (5), HepB vaccine birth dose coverage was 36%. These findings highlight the global need to implement this key hepatitis B prevention strategy more widely. Since 1998, WHO and UNICEF have used the Joint Reporting Form to collect information annually from WHO member states on coverage and indicators of immunization system performance for all WHO-recommended infant vaccines (4). For HepB vaccine, information is collected about the schedule used, the number of infants receiving the recommended 3 doses of vaccine, and (for those countries where the national immunization schedule includes a HepB vaccine birth dose) the administrative coverage of HepB vaccine birth dose. As of 2006, 81 (42%) of 193 WHO member states indicated that a HepB vaccine birth dose was included in the national infant immunization schedule. Of the 87 countries where chronic HBV infection prevalence has been high historically (>8%), 38 (44%) reported including a HepB vaccine birth dose in their immunization schedules (Table 1). Of the 135.0 million infants born worldwide in 2006, 62.7 million infants were born in countries where chronic HBV infection prevalence has been high historically. Global and regional HepB vaccine birth dose coverage were calculated using reported coverage figures from the Joint Reporting Form and estimates of the number of live births (6). In this analysis, countries that did not report birth dose coverage on the Joint Reporting Form were assumed to have 0% birth dose coverage. Among the 81 countries reporting a HepB vaccine birth dose in their immunization schedules, 22 (27%) did not report birth dose coverage data. As a result, 11%--20% of the birth cohort might have received a HepB vaccine birth dose but was assumed to have 0% coverage because of lack of reporting. Birth dose coverage worldwide was 27% and varied widely by region, from 3% to 71% (Table 2). Birth dose coverage for countries with >8% chronic HBV infection prevalence was 36% (range by region: 1%--92%), and for countries with <8% prevalence was 20% (range by region: 0%--97%) (Table 2). However, in response to an open-ended Joint Reporting Form question regarding which vaccination schedule was used, several member states indicated that a first dose administered beyond 24 hours of birth still could be considered a birth dose. Reported by: L Dumolard, PhD, M Gacic-Dobo, CN Shapiro, MD, S Wiersma, MD, Immunization, Vaccines, and Biologicals, Expanded Programme on Immunization, World Health Organization, Geneva, Switzerland. SA Wang, MD, Div of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. Editorial Note:This report presents the first analysis of WHO-UNICEF Joint Reporting Form data that estimates worldwide HepB vaccine birth dose coverage. HepB vaccine birth dose coverage during 2006 was 27% globally and 36% for children born in countries where chronic HBV infection has been highly endemic (>8%). The relatively low coverage is consistent with survey data from several countries (7) and suggests that program performance for newborn HepB vaccination needs improvement. Two major modes of HBV transmission occur during infancy: 1) from an infected mother to her newborn during delivery, and 2) from an infected household contact to the infant. Perinatal HBV transmission accounts for an estimated 21% of HBV-related deaths globally and 13%--26% regionally (1). HepB vaccine is 70%--95% effective as postexposure prophylaxis in preventing mother-to-infant HBV transmission when the first dose is administered within 24 hours after birth (8). HepB vaccination of newborns also provides early preexposure protection to infants born to uninfected women during a period when, if HBV exposure were to occur, the risk for developing chronic HBV infection is greatest (i.e., during the first year of life). Infants who become HBV infected have an approximately 90% risk for developing chronic HBV infection, and when chronically infected, have a 25% risk for dying prematurely from cirrhosis or liver cancer. Thus, newborn HepB immunization is a key intervention to prevent perinatal HBV transmission and a critical strategy to reduce the global morbidity and mortality associated with hepatitis B. When introducing HepB vaccine into infant immunization programs, national policy makers must decide when to begin the HepB vaccine series: 1) at birth for all infants, 2) at birth, but targeted only to newborns of HBV-infected women, or 3) at the same time in the immunization schedule as other vaccines are administered to all infants (e.g., at 6 weeks, when national immunization programs in most developing countries initiate administration of other vaccines to infants) but at a time that is too late to prevent perinatal HBV infection. Administering a HepB vaccine birth dose only to newborns of HBV-infected women usually is not feasible in developing countries where hepatitis B is highly endemic (3), is a practice that is prone to error and results in missed postexposure prophylaxis of infants (even in countries where testing and identifying infected women during pregnancy is well established) (8), and fails to provide early preexposure protection to newborns of uninfected women who might have household contacts who are infected. In the WHO Western Pacific region (where hepatitis B is highly endemic in many countries), 23 of 26 countries have introduced HepB vaccine starting at birth. However, countries with >8% endemic chronic HBV infection in other regions might not have introduced a HepB vaccine birth dose because disease burden from perinatal HBV transmission was not believed to be significant or because of challenges in implementing the birth dose (1,9). Challenges to administering HepB vaccine to newborns within 24 hours after birth can be logistical and financial. First, many infants, especially in remote or poor areas, are born at home and do not have access at birth to skilled attendants who can administer vaccinations. Increasing the number of infants born in facilities or attended by trained health staff would improve birth dose coverage. Second, infant vaccinations usually are administered by vaccination providers in well-baby clinics or other outpatient health settings or during outreach immunization sessions in the community, whereas care of the mothers during delivery and of infants immediately after birth often is provided by maternal health workers, so coordination of these two types of workers is needed. Third, in many parts of the world, vaccines are transported and delivered in cold storage boxes at monthly or even longer intervals from a central source to locations where they will be administered and can only be stored for several days. As a result, HepB vaccine might not be available when infants are born at more remote facilities. Improving the range of the cold storage delivery chain and exploring options for making vaccine available outside that range are needed. Fourth, many developing countries have modified their immunization schedules to include new multivalent vaccines (e.g., combined Haemophilus influenzae type b and HepB vaccine) that cannot be administered to newborns. These vaccines provide antigens against two or more diseases and are supported by international donors, but the countries must rely on their own limited resources to purchase separately the monovalent HepB vaccine necessary for the HepB vaccine birth dose. Finally, awareness among providers and parents about the importance of administering HepB vaccine within 24 hours of birth often is lacking, so health promotion and training are needed (9,10). The findings in this report are subject to at least two limitations. First, coverage data were missing from 27% of countries that reported having HepB vaccine birth dose in their immunization schedules, and for which an assumption was made of 0% birth dose coverage. Second, HepB vaccine birth dose coverage reported by countries sometimes might have included doses administered after 24 hours of birth, as indicated by the response on the Joint Reporting Form from several countries that a first dose administered beyond 24 hours of birth would be considered a birth dose. This lack of understanding as to what constitutes an appropriate birth dose of HepB vaccine might reflect the fact that the term "birth dose" also is used widely for bacille Calmette-Guérin vaccine administration a few weeks after birth, and for oral poliovirus vaccine administration several days after birth. Data on HepB vaccine birth dose coverage from the Joint Reporting Form could be validated by using national coverage surveys that compare date of birth with date of administration of the first HepB vaccine dose for infants. More complete implementation of routine newborn HepB vaccination globally would reduce the substantial morbidity and mortality caused by perinatally acquired HBV infection. Newborn HepB vaccination is of highest priority in highly endemic areas where the contribution of perinatal transmission to the overall disease burden is greatest. However, even in countries with <8% chronic HBV infection prevalence, newborn HepB vaccination can be an important hepatitis B control strategy. Disease modeling suggests that implementing HepB vaccine birth dose in regions with relatively low prevalence of chronic HBV infection, such as the Americas or Europe, might reduce HBV mortality by an additional 10%--20% compared with following a HepB vaccination schedule without a birth dose (1). For this reason, a substantial number of countries in areas with intermediate or low hepatitis B endemicity have implemented newborn HepB vaccination. References
Table 1 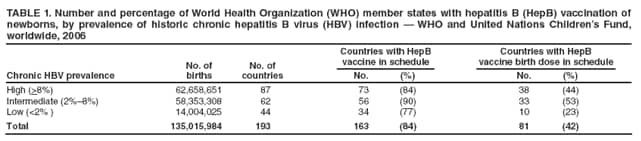 Return to top. Table 2 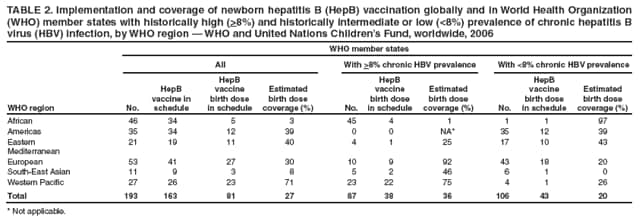 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 11/20/2008 |
|||||||||
|