 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
| Weekly |
| August 10, 2007 / 56(31);785-789 |
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Dengue Hemorrhagic Fever --- U.S.-Mexico Border, 2005Please note: An erratum has been published for this article. To view the erratum, please click here. Dengue fever is a mosquito-transmitted disease caused by any of four closely related virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4) of the genus Flavivirus. Infection with one of these serotypes provides lifelong immunity to the infecting serotype only. Therefore, persons can acquire a second dengue infection from a different serotype, and second infections place them at greater risk for dengue hemorrhagic fever (DHF), the more severe form of the disease (1). DHF is characterized by bleeding manifestations, thrombocytopenia,* and increased vascular permeability that can lead to life-threatening shock (2). In south Texas, near the border with Mexico, sporadic, locally acquired outbreaks of dengue fever have been reported previously; however, on the Texas side of the border, these outbreaks have not included recognized cases of locally acquired DHF in persons native to the area. In July 2005, a case of DHF was reported in a resident of Brownsville, Texas (Figure 1). In August 2005, health authorities in the neighboring state of Tamaulipas, Mexico, reported an ongoing dengue outbreak with 1,251 cases of dengue fever, including 223 cases (17.8%) of DHF. To characterize this dengue outbreak, the Texas Department of State Health Services (TDSHS), Mexican health authorities, and CDC conducted a clinical and epidemiologic investigation. This report summarizes the results of that investigation, which determined that the percentage of DHF cases associated with dengue fever outbreaks at the Texas-Tamaulipas border has increased. Health-care providers along the U.S. border with Mexico should be vigilant for DHF and familiar with its diagnosis and management to reduce the number of severe illnesses and deaths associated with outbreaks of dengue fever. Autochthonous DHF Case ReportOn June 24, 2005, a woman from Brownsville, Texas, had acute onset of fever, chills, headache, nausea, vomiting, abdominal pain, arthralgia, and myalgia. As a youth, the patient had resided across the border in the city of Matamoros in Tamaulipas, Mexico; however, she had been a Brownsville resident for 16 years with the exception of 1 year in Houston, Texas. After she became ill, the woman crossed the border into Matamoros for the first time in approximately 2 months, where she visited a clinician and was given antibiotics. On June 28, the woman was hospitalized in Matamoros with a diagnosis of probable dengue fever and urinary tract infection. During her 3-day hospitalization in Mexico, she had thrombocytopenia (62,000 platelets/mm3) but no hemorrhagic manifestations; she was treated with fluids and antibiotics and discharged. On July 1, the woman reentered the United States and sought treatment for continued fever, chills, vomiting, and abdominal pain. She was admitted to a hospital in Brownsville, Texas, where her blood pressure was 94/70 mm Hg, and laboratory testing indicated proteinuria, hematuria, and a further decrease in platelet count (43,000/mm3). She was given antibiotics for suspected partially treated urinary tract infection and fluids for dehydration. During her hospital stay, the patient's platelet count dropped to 39,000/mm3 and albumin to 2.9 g/100 mL; a fecal occult blood test was positive, and pleural effusion was noted on ultrasound. Upon discharge on July 4, her platelet count had increased to 118,000/mm3. The woman was discharged with a diagnosis of possible murine typhus or viral infection and instructions to take a course of doxycycline. Although the woman's clinical characteristics (i.e., acute fever, platelet count <100,000/mm3, evidence of bleeding [hematuria and fecal occult blood] and plasma leakage) were consistent with World Health Organization (WHO) criteria for DHF (Box) (2), dengue was not diagnosed at the Brownsville hospital. Subsequently, results from a July 3 serum sample from the woman obtained by the regional Texas Border Infectious Disease Surveillance (BIDS) project tested positive for dengue immunoglobulin M (IgM) by enzyme-linked immunosorbent assay (ELISA) and had an elevated titer of immunoglobulin G (IgG) antibodies to dengue fever (1:655,350); this was interpreted as indicative of a secondary dengue infection (1). Outbreak Investigation and ResponseDengue fever case finding. On August 27, 2005, Tamaulipas State Health Services reported to TDSHS that an outbreak of dengue fever in the border state had grown to 1,251 cases that met the Mexico case definition (i.e., fever and at least two of the following symptoms: headache, myalgia, arthralgia, and rash). Using WHO criteria for DHF, Tamaulipas health authorities had classified 223 (17.8%) of the cases as DHF, an increase in the percentage classified as DHF from 2000--2004, when 541 dengue fever cases were reported, including 20 cases (3.7%) classified as DHF.† In October, investigators in Texas and Tamaulipas began conducting expanded outbreak case finding, including active surveillance in local hospitals, with laboratory testing encouraged for patients with undifferentiated fever as part of the BIDS project. In Cameron County, Texas, where Brownsville is the county seat, TDSHS identified 24 additional cases of laboratory-confirmed dengue fever§, including two additional cases of locally transmitted dengue fever and 22 cases associated with travel to Mexico; the cases had been reported during August--November (Figure 2). The serotype most commonly associated with the outbreak was identified as DEN-2 (i.e., 27 of 28 viral isolates in Tamaulipas). Molecular analysis of isolates at CDC indicated that the circulating strain of DEN-2 was one previously associated with DHF in the Americas region (4,5). Plotting reports of cases by week determined that the border outbreak peaked in October and substantially subsided by December (Figure 2). DHF case finding. In December, investigators reviewed medical records of 129 patients who had been hospitalized and reported to public health authorities with both clinical and laboratory evidence of dengue fever, including 25 persons treated at three Cameron County hospitals and 104 treated at three hospitals in Matamoros. Fifty-nine percent of the patients were female. Ages ranged from 30 to 76 years (median 47.5 years) among the Cameron County cases and from 7 to 70 years (median 36.0 years) among the Matamoros cases. In addition to fever, 82% had myalgia, 78% headache, 41% abdominal pain, 23% rash, and 19% had underlying chronic diseases. No fatalities were recorded. A total of 16 (64.0%) of the 25 dengue cases from Cameron County and 34 (32.7%) of the 104 cases from Matamoros met WHO criteria for DHF (Box). Eleven of the 50 DHF cases, including one from Cameron County, were classified as WHO grade III, or dengue shock syndrome, with early or mild evidence of hypotension or shock. The remaining 39 DHF cases were classified as WHO grade II.¶ Serosurveys. Because many dengue infections are asymptomatic, and most ill persons likely do not seek medical attention, investigators conducted serosurveys to assess the incidence of dengue infection in the populations of Matamoros and Brownsville. Serosurveys also enable estimation of the population susceptible to second dengue infections and DHF. For the serosurveys, a two-stage cluster design was used to obtain a representative sample of households from Brownsville and Matamoros (6). Thirty census tracts were selected systematically from each city after stratifying by income. Four households were selected from each census tract after mapping and selecting a random start point and random direction for sampling. At each participating household, all residents present and aged >5 years were asked to provide a blood sample and demographic information. Serum samples were tested for IgM and IgG antibodies to dengue virus by ELISA. The seroincidence of recent dengue infection was defined by IgM antibodies >0.2 optical density (OD). Seroprevalence was defined as the presence of IgG antibodies >1:40. Data were weighted to reflect probability of selection, taking into account the population and numbers of households per census tract and size of household. In Matamoros, 240 households were visited during December 5--10, and 143 (59.6%) had residents at home. Blood samples were obtained from 131 persons in 111 homes. Of these samples, 30 were anti-dengue IgM positive (weighted prevalence: 22.8%; 95% confidence interval [CI] = 13.3%--32.3%), and 101 were IgG positive (weighted prevalence: 76.6%; CI = 64.7%--88.5%). In Brownsville, 346 households were visited during December 12--15, and 161 (46.5%) had residents at home. Blood samples were obtained from 141 persons in 118 homes. Of these samples, four were anti-dengue IgM positive (weighted prevalence: 2.5%; CI = 0%--5.4%) and 47 were IgG positive (weighted prevalence: 38.2%; CI = 26.7%--49.8%). Of 24 Brownsville participants with no history of travel outside the United States, six (25%) were seropositive for IgM or IgG antibodies to dengue. Reported by: A Abell, PhD, B Smith, MD, M Fournier, MD, Texas Dept of State Health Svcs, Harlingen, Texas; T Betz, MD, L Gaul, PhD, Texas Dept of State Health Svcs, Austin, Texas; JL Robles-Lopez, MD, CA Carrillo, MD, Jurisdicción Sanitaria No. III de Matamoros, Matamoros, Tamaulipas; A Rodríguez-Trujillo, MD, Servicios de Salud de Tamaulipas, Cd. Victoria, Tamaulipas; C Moya-Rabelly, MD, Mexico Section of the US-Mexico Border Health Commission, Tijuana, Baja California; O Velasquez-Monroy, MD, C Alvarez-Lucas, MD, Centro Nacional de Vigilancia Epidemiológica y Control de Enfermedades, Mexico, DF; P Kuri-Morales, MD, L Anaya-Lopez, MD, Dirección General de Epidemiología, México, DF. M Hayden, PhD, National Center for Atmospheric Research, Boulder, Colorado. E Zielinski-Gutierrez, DrPH, J Muñoz, PhD, M Beatty, MD, I Sosa, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; S Wenzel, MPH, Career Development Div, Office of Workforce and Career Development; M Escobedo, MD, S Waterman, MD, Div of Global Migration and Quarantine, National Center for Preparedness, Detection, and Control of Infectious Diseases; M Ramos, MD, BK Kapella, MD, H Mohammed, PhD, R Taylor, PhD, J Brunkard, PhD, EIS officers. Editorial Note:DHF incidence has increased in the Western Hemisphere in Latin America and the Caribbean during the past two decades (3). Over this period, the epidemiology of dengue in Mexico and Texas has changed. Since 1995, when all four dengue serotypes were identified as circulating in Mexico, an increasing percentage of reported dengue cases in Mexico have been DHF (7). In the Mexican border state of Tamaulipas, all four serotypes were first reported in circulation in 1995, and the proportion of reported DHF cases increased from 2.2% in 2000 to 23.4% in 2006. In south Texas, all dengue serotypes have circulated periodically (3,8), but locally acquired DHF has been reported only recently (9). The first report of locally acquired DHF in Texas, published in 2004, described a fatal case involving a woman originally from Southeast Asia (9). She presumably had acquired her first dengue infection in Asia and her second dengue infection in Val Verde, Texas, near the U.S.-Mexico border. However, the DHF case described in this report is the first in a Texas resident who was native to the U.S.-Mexico border area. Case-finding activities during the dengue outbreak identified 15 additional DHF cases on the Texas side of the border. Entomologic, serologic and virologic conditions are now such that locally acquired DHF can occur in south Texas. The principal dengue vector, the Aedes aegypti mosquito, is well established in south Texas, as is Aedes albopictus, which also is capable of transmitting dengue (7,10; TDSHS, unpublished data, 2007). The finding that 38% of surveyed Brownsville residents have IgG antibodies to dengue indicates that a substantial proportion of the city population has been infected with the dengue virus and might be more susceptible to DHF if they receive a second infection with a heterologous dengue serotype. The presence in Brownsville of multiple dengue serotypes since 1980 might increase the likelihood for secondary dengue infections from a different serotype and increase the risk for DHF. The findings in this report are subject to at least two limitations. First, more comprehensive laboratory testing on the U.S. side of the border during the 2005 outbreak likely accounted for the greater percentage of patients meeting DHF criteria among hospitalized dengue patients in Cameron County compared with Matamoros. As such, the results for these two sites are not directly comparable. Second, because anti-dengue IgM antibodies do not always remain elevated 2--3 months after infection, especially after a second infection, the serosurvey conducted during December 5--15 likely underestimated the number of recent dengue infections in Brownsville and Matamoros. Health authorities along the Texas-Tamaulipas border should consider strengthening surveillance for dengue fever, given the potential for future outbreaks with increased risk for DHF. Maintaining active virologic surveillance for circulating serotypes also is important to provide early warning of possible epidemics. Clinicians in the south Texas area and members of the public should be aware of the potential for DHF in addition to dengue fever in the region. Furthermore, clinicians should be trained to recognize and manage DHF. Early recognition and diagnosis of DHF and careful fluid management can reduce the case fatality rate in cases with shock to less than 1%. Public health officials should continue outreach activities to advise communities of prevention measures, including effective mosquito surveillance and reduction programs. Acknowledgments This report is based, in part, on contributions from DJ Gubler, Asia-Pacific Institute of Tropical Medicine and Infectious Diseases, Honolulu, Hawaii; J Ramirez, City of Brownsville Public Health Dept, Texas; R Burton, Texas Dept of State Health Svcs; and state and local health departments in Texas and Tamaulipas, Mexico. References
* <100,000 platelets/mm3. † Boletín Epidemiolgía [Spanish] México, D.F. Dirección General de Epidemiología, 2000--2006. Available at http://www.dgepi.salud.gob.mx/boletin/boletin.htm. § Defined as the presence of anti-dengue IgM antibody, dengue viral identification by polymerase chain reaction, or virus isolation from a blood sample of a patient with clinically compatible symptoms. ¶ DHF is classified into four grades of severity; grades III and IV are considered to be dengue shock syndrome. Grade I: Fever accompanied by nonspecific constitutional symptoms; the only hemorrhagic manifestation is a positive tourniquet test and/or easy bruising. Grade II: Spontaneous bleeding in addition to the manifestations of Grade I patients, usually in the forms of skin or other hemorrhages. Grade III: Circulatory failure manifested by a rapid, weak pulse and narrowing of pulse pressure or hypotension, with the presence of cold, clammy skin and restlessness. Grade IV: Profound shock with undetectable blood pressure or pulse (2).
Figure 1 Return to top. Figure 2 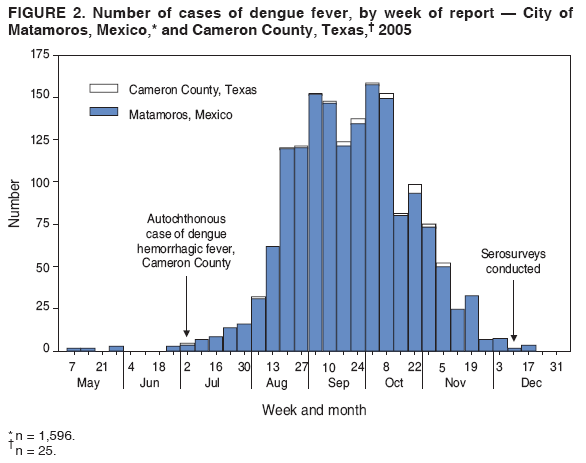 Return to top. Box 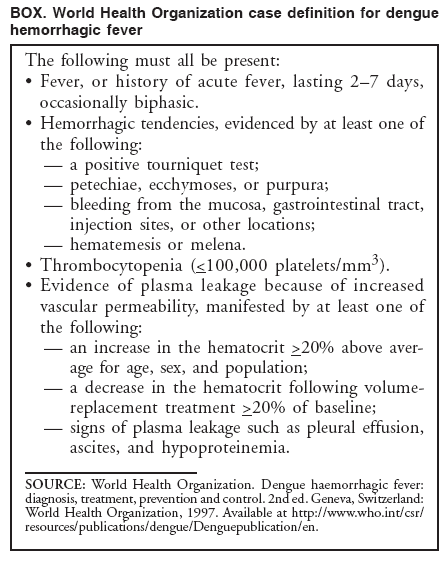 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 8/8/2007 |
|||||||||
|