 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
| Weekly |
| November 9, 2001 / 50(44);973-6 |
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Investigation of Bioterrorism-Related Anthrax and Adverse Events from Antimicrobial ProphylaxisCDC and state and local public health authorities continue to investigate cases of bioterrorism-related anthrax. As of November 7, a total of 22 cases of anthrax have been identified according to the CDC surveillance case definition; 10 were confirmed inhalational anthrax cases and 12 cases (seven confirmed and five suspected) were cutaneous anthrax (Table 1). The majority of cases have occurred in persons working at postal facilities in New Jersey (NJ) and the District of Columbia (DC) in which letters contaminated with anthrax were handled or processed using high-speed sorting machines, or at media companies in New York City (NYC) or Florida (FL) where letters, either confirmed or presumed to be contaminated with anthrax, were opened or handled. The probable exposures for a case of cutaneous anthrax in NJ and a case of inhalational anthrax in NYC remain unknown. Epidemiologic investigations of these cases and surveillance to detect new cases of bioterrorism-associated anthrax continue. This report updates the investigation of these cases and describes adverse events associated with antimicrobial prophylaxis. Since the last report (1), one additional case of confirmed cutaneous anthrax has been identified in a 38-year-old man who worked at a media company in NYC. This is the third case of cutaneous anthrax reported among employees at the media company and is probably associated with a contaminated letter postmarked September 18 that was handled during October 12--15. On October 23, the patient noted a small nonerythematous, nonpruritic, and painless lesion on his forehead. On October 28, a physician evaluated the patient and described a lesion 1.4 cm in diameter, the center of which was depressed and dark gray; the same day, he was started on ciprofloxacin. A biopsy was positive for Bacillus anthracis by culture and immunohistochemical staining. No other new cases have been identified from investigations in FL, DC, NJ, NYC, or other areas. Recommendations for antimicrobial prophylaxis to prevent inhalational anthrax have been directed by epidemiologic and laboratory findings. Approximately 300 postal and other facilities have been tested for B. anthracis spores and approximately 32,000 persons have initiated antimicrobial prophylaxis following potential exposure to B. anthracis at workplaces in FL, DC, NJ, and NYC. Clean-up at contaminated sites and surveillance for new anthrax cases are ongoing. Adverse Events from Antimicrobial ProphylaxisDuring October 8--10, a total of 1,132 persons from company A in Boca Raton, Florida, received initial antimicrobial prophylaxis for presumed exposure to B. anthracis; 970 (86%) persons received ciprofloxacin. After 14 days of prophylaxis, of 1,000 persons for whom information was available, 797 (80%) were still taking antibiotics. A questionnaire was administered on approximately day 7 or day 14 of prophylaxis to assess adverse events in 490 (62%) persons who reported taking antibiotics. Of 490 persons, 95 (19%) reported one or more of the following symptoms: itching; breathing problems; swelling of face, neck, or throat; or seeking medical attention for any adverse events related to taking the antibiotic. Clinic record review and telephone interviews of the 95 indicated that six persons reported seeking medical attention and did not continue taking their original medication, possibly because of adverse events. A detailed questionnaire was administered to these six persons to determine the temporal association between initiation of antimicrobial prophylaxis and symptom onset, medical care received, and underlying illnesses. Two persons had been seen by a physician as outpatients, two had been seen in emergency departments, and two had been hospitalized. None of the persons had documented objective findings or clinical history attributable to adverse events, including anaphylaxis (2). Similar screening for adherence to and adverse events associated with antimicrobial prophylaxis has been initiated in DC, NJ, and NYC. Public Health ResponseCDC and local health departments continue to respond to public concern and bioterrorism threats. During October 8--31, CDC's Emergency Operations Center received 8,860 telephone inquiries from all 50 states, Puerto Rico, Guam, and 22 foreign countries. Of these, 590 (6.7%) calls were thought to represent a potential threat as defined by a report of exposure to a substance possibly associated with bioterrorism or symptoms consistent with an illness associated with bioterrorism. The 590 calls regarding potential threats were from physicians or other health-care workers (40%); local or state health departments (14%); private citizens (14%); and police, fire, or emergency response departments (7%). In response to the calls, CDC has provided information; referred to appropriate local, state, or federal agencies; assisted with clinical diagnosis or management; or initiated additional epidemiologic investigations of illnesses compatible with bioterrorism. State and local public health agencies also are addressing public concerns and investigating potential bioterrorist threats. CDC has established a secured web-based system for states to report weekly summaries of their bioterrorism-related activities. For the week of October 21--27, Colorado, Connecticut, Louisiana, Maryland, Montana, North Dakota, Tennessee, Wisconsin, and Wyoming reported 2,817 bioterrorism-related calls (mean per state: 313; range: 23--800) and approximately 25 investigations of bioterrorism threats in each state. From eight to 30 full-time personnel are engaged in these responses in each state. For the same period, public health laboratories in 46 states participating in the Laboratory Response Network reported receiving approximately 7,500 specimens and isolates for B. anthracis testing. These specimens were primarily from environmental samples and nasal swabs. Reported by: J Malecki, MD, Palm Beach County Health Dept, Palm Beach; S Wiersma, MD, State Epidemiologist, Florida Dept of Health. R Labinson, MD, L Kamal, MD, St. Clare's Hospital and Health Center, New York, New York; New York City Dept of Health. E Bresnitz, MD, State Epidemiologist, G DiFerdinando, MD, New Jersey Dept of Health and Senior Svcs. P Lurie, MD, K Nalluswami, MD, Pennsylvania Dept of Health. L Hathcock, PhD, State Epidemiologist, Delaware Div of Public Health. L Siegel, MD, S Adams, I Walks, MD, J Davies-Coles, PhD, M Richardson, MD, District of Columbia Dept of Health. R Brechner, State Epidemiologist, Maryland Dept of Health and Hygiene. R Stroube, MD, State Epidemiologist, Virginia Dept of Health. US Dept of Defense. EIS officers, CDC. Editorial Note:Since the last report, one new case of confirmed cutaneous anthrax has been identified in a media company employee resulting from exposure to a previously known contaminated letter. The probable source of exposure for two cases reported last week (one cutaneous and one inhalational) has yet to be determined. Although these two cases ultimately might be attributed to letter handling, the lack of a discernable link to previous cases or workplaces raises the possibility of new routes of exposure or new target populations. Since October 8, approximately 32,000 persons with potential exposure to B. anthracis in FL, NJ, NYC, and DC have initiated antimicrobial prophylaxis to prevent anthrax infection, and for approximately 5,000 persons, a 60-day course of antibiotics has been recommended. The Code of Federal Regulations* defines a serious adverse event associated with using a biologic product in humans as any of the following: death, life-threatening adverse event, inpatient hospitalization or prolongation of an existing hospitalization, persistent or substantial disability/incapacity, congenital anomaly/birth defect, or an important medical event that requires medical or surgical intervention to avert one of these outcomes. Although two persons were hospitalized in FL, their illnesses were not associated with antimicrobial prophylaxis. Efforts to contact persons who have not yet received followup are ongoing. Adverse events associated with ciprofloxacin and doxycycline have been well-described among patients taking these medications for short-term treatment of bacterial infections. Anaphylactoid reactions caused by drug reaction have been reported rarely (3). However, few data exist regarding the use of these antimicrobials for longer periods. Because many persons are receiving antimicrobial prophylaxis, enhanced surveillance programs are essential to detect and monitor adverse events associated with these medications. Moreover, these programs will monitor adherence to the full 60-day regimen, enabling the design of better programs to promote completion of recommended prophylactic regimens. CDC and state and local public health agencies are continuing epidemiologic and laboratory investigations of bioterrorism-related anthrax. Even without confirmed cases of anthrax, state and local health departments have responded to public concerns and have applied substantial personnel and laboratory resources to address anthrax issues in recent weeks. Recent cases of anthrax are attributed to intentional infection of persons and represent criminal acts that are being investigated by federal law enforcement agencies. Because new cases of anthrax may occur, public health authorities and clinicians should remain vigilant. References
* 21 CFR 600.80. Table 1 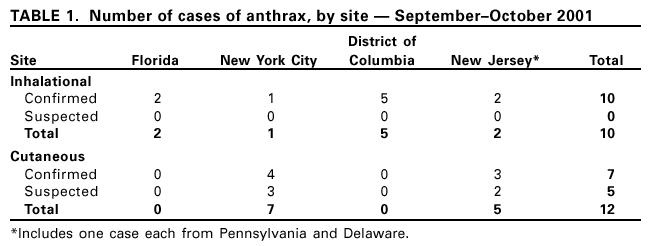 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/13/2001 |
|||||||||
This page last reviewed 11/13/2001
|