Summary: ‘Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2023-24’
- Groups Recommended for Vaccination
- Timing of Vaccination
- Approved Ages & Dose Volumes
- Influenza Vaccination in Pregnancy
- Number of Doses for Ages 6 Months through 8 Years
- Adults Aged ≥65 Years
- Vaccination of Persons with COVID-19
- Persons with Chronic Medical Conditions
- Immunocompromised Persons
- Caregivers and Contacts of High-Risk Persons
- Persons with Egg Allergy
- Previous Severe Allergic Reactions to Influenza Vaccines
- Vaccination Issues for Travelers
- Vaccination and Influenza Antiviral Medications
- Administration of Influenza Vaccines with Other Vaccines
- Vaccine Adverse Event Reporting System (VAERS)
- Further Information
- Inactivated Influenza Vaccines (IIV4s) and Recombinant Influenza Vaccine (RIV4)
- Live Attenuated Influenza Vaccine (LAIV4)
- Influenza Vaccine Contraindications and Precautions
- Contraindications and Precautions for Persons with a History of Severe Allergic Reaction to an Influenza Vaccine
For additional information: MMWR Recomm Rep 2023;72 (No. RR-2) at https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/flu.html
Groups Recommended for Vaccination
- Routine annual influenza vaccination is recommended for all persons aged ≥6 months who do not have contraindications.
- If supply is limited, see priority groups in the ACIP statement.
- For most persons who need only one dose of influenza vaccine for the season, vaccination should ideally be offered during September or October. However, vaccination should continue throughout the season as long as influenza viruses are circulating.
- Vaccination during July and August is not recommended for most groups. Timing considerations include:
- For most adults (particularly those aged ≥65 years) and pregnant persons in the first or second trimester, vaccination during July and August should be avoided unless there is concern that later vaccination might not be possible.
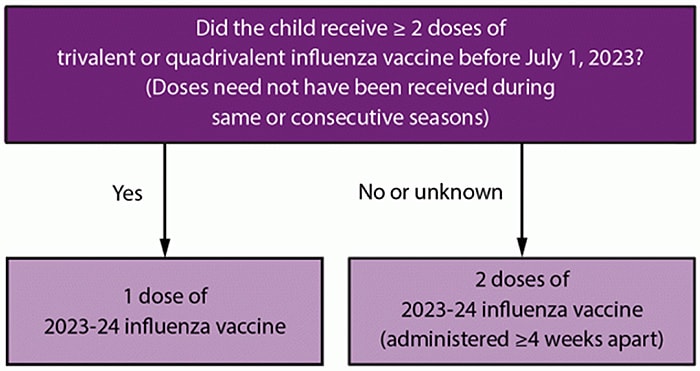
- Children 6 months through 8 years who require 2 doses (Figure) should receive the first dose as soon as vaccine is available.
- Vaccination during July and August can be considered for children of any age who require only 1 dose.
- Vaccination in July and August can be considered for pregnant persons who are in the third trimester during these months (see also Influenza Vaccination in Pregnancy).
Approved ages and dose volumes for intramuscular influenza vaccines (IIV4s and RIV4):
| Vaccine | Approved Ages | Dose volume |
|---|---|---|
| Afluria Quadrivalent | 6 through 35 months
≥3 years |
0.25 mL
0.5 mL |
| Fluarix Quadrivalent | ≥6 months | 0.5 mL |
| FluLaval Quadrivalent | ≥6 months | 0.5 mL |
| Fluzone Quadrivalent | 6 through 35 months
≥3 years |
0.5 mL (see below)
0.5 mL |
| Flucelvax Quadrivalent | ≥6 months | 0.5 mL |
| Flublok Quadrivalent | ≥18 years | 0.5 mL |
| Fluzone High-Dose Quadrivalent | ≥65 years | 0.7 mL |
| Fluad Quadrivalent | ≥65 years | 0.5 mL |
- The approved dose volume per the package insert for Fluzone Quadrivalent is either 0.25 mL or 0.5 mL for ages 6 through 35 months. However, 0.25mL prefilled syringes are not available.
- If a dose less than the necessary volume is administered:
- If the error is discovered immediately (before the recipient has left the vaccination setting), administer the remaining additional volume needed.
- If it is difficult to measure the remaining needed volume, or if the error is discovered after the recipient has left the vaccination setting, administer a repeat full dose.
- Healthy non-pregnant persons aged 2 through 49 years may alternatively receive 0.2 mL of LAIV4, 0.1 mL per nostril, using the supplied intranasal sprayer (Table 3).
- Determine the number of doses needed based on child’s age at time of first dose of 2023–24 influenza vaccine and number of doses of influenza vaccine received in previous seasons (Figure).
- Children aged 6 months through 8 years who have previously received ≥2 total doses of trivalent or quadrivalent influenza vaccine ≥4 weeks apart before July 1, 2023 need 1 dose of 2023-24 influenza vaccine. The two previous doses do not need to have been received in the same or consecutive influenza seasons.
- Children aged 6 months through 8 years who have not previously received ≥2 total doses of trivalent or quadrivalent influenza vaccine ≥4 weeks apart before July 1, 2023 or whose influenza vaccination history is unknown need 2 doses of 2023-24 influenza vaccine, given ≥4 weeks apart.
- For children aged 8 years who require 2 doses, both doses should be administered even if the child turns age 9 years between dose 1 and dose 2.
- Persons aged ≥9 years need only one dose.
- ACIP recommends that adults aged ≥65 years preferentially receive any one of the following higher dose or adjuvanted influenza vaccines: quadrivalent high-dose inactivated influenza vaccine (HD-IIV4), quadrivalent recombinant influenza vaccine (RIV4), or quadrivalent adjuvanted inactivated influenza vaccine (aIIV4). If none of these three vaccines is available at an opportunity for vaccine administration, then any other age-appropriate influenza vaccine should be used.
- Data support greater potential benefit of HD-IIV3, aIIV3, or RIV4 relative to standard-dose unadjuvanted IIVs in this age group, with the most data available for HD-IIV3; but comparisons of these vaccines with one another are limited.
- Vaccination of moderately or severely ill persons should be deferred until recovery from the acute illness. For persons who are mildly ill or asymptomatic, deferral might be considered to avoid confusing illness symptoms with vaccine reactions.
- Other considerations include current influenza activity, the recipient’s risk of severe influenza illness, use of immunosuppressive agents that might blunt immune response, and risk of exposing others in the vaccination setting.
- LAIV4 is not recommended for persons with some chronic medical conditions (Table 3).
- Immunocompromised persons should receive an age-appropriate IIV4 or RIV4. LAIV4 should not be used.
- Immune response might be reduced in persons on certain medications, chemotherapy, or transplant regimens.
- Timing influenza vaccination relative to a specified period before or after interventions that compromise immunity may be appropriate. The Infectious Diseases Society of America (IDSA) has published guidance concerning the timing of vaccination in relation to such interventions (see Further Information).
- Caregivers and contacts (including those of immunosuppressed persons) may receive any age-appropriate IIV4 or RIV4.
- LAIV4 may be given to caregivers and contacts of persons who are not severely immunocompromised (i.e., who do not require a protected environment).
- Health care personnel or hospital visitors who receive LAIV4 should avoid caring for/contact with severely immunosuppressed persons who require a protected environment for 7 days after vaccination.
- All persons ages ≥6 months with egg allergy should receive influenza vaccine. Any influenza vaccine (egg based or non-egg based) that is otherwise appropriate for the recipient’s age and health status can be used.
- Egg allergy necessitates no additional safety measures for influenza vaccination beyond those recommended for any recipient of any vaccine, regardless of severity of previous reaction to egg.
- Severe and life-threatening reactions to vaccines can occur with any vaccine and in any vaccine recipient, regardless of allergy history. All vaccines should be administered in settings in which personnel and equipment needed for rapid recognition and treatment of acute hypersensitivity reactions are available.
- Previous severe allergic reaction (e.g., anaphylaxis) to any influenza vaccine (any egg-based IIV, ccIIV, RIV, or LAIV of any valency) is a contraindication to all egg-based IIV4s and LAIV4.
- Previous severe allergic reaction to ccIIV of any valency or to any component of ccIIV4 is a contraindication to ccIIV4. Previous severe allergic reaction to any other influenza vaccine (any egg-based IIV, RIV, or LAIV of any valency) is a precaution to ccIIV4.
- Previous severe allergic reaction to RIV of any valency or any component of RIV4 is a contraindication to RIV4. Previous severe allergic reaction to any other influenza vaccine (any egg-based IIV, ccIIV, or LAIV of any valency) is a precaution to RIV4.
- Each vaccine is also contraindicated for those with a history of severe allergic reaction to any component of that vaccine (other than egg; see Persons with Egg Allergy).
- See Table 3 and Table 4 for more information.
- Travelers who wish to reduce risk for influenza should consider vaccination, preferably ≥2 weeks before departure.
- Persons at higher risk for complications of influenza who were not vaccinated during the preceding fall or winter should consider influenza vaccination before departure, if planning to travel to the tropics, with organized tourist groups, on cruise ships, or to the Southern Hemisphere during April-September.
- Southern Hemisphere influenza vaccines might differ in viral composition from Northern Hemisphere formulations.
- Vaccination with Southern Hemisphere influenza vaccine prior to Southern Hemisphere travel might be reasonable; however, these formulations are generally not available in the U.S.
- IIV4 and RIV4 may be administered to persons receiving influenza antiviral medications.
- Influenza antivirals might reduce effectiveness of LAIV4, if given before or after LAIV4. Persons who receive influenza antivirals during the following periods should be revaccinated with an age-appropriate IIV4 or RIV4 (intervals may be longer in conditions where medication clearance is delayed):
| Influenza Antiviral | Estimated window for potential LAIV interference (based upon half-life reported in package insert) |
|---|---|
| Oseltamivir and Zanamivir | 48 hours before to 2 weeks after LAIV4 |
| Peramivir | 5 days before to 2 weeks after LAIV4 |
| Baloxavir | 17 days before to 2 weeks after LAIV4 |
- IIV4s and RIV4 may be administered concurrently or sequentially with other live or inactivated vaccines.
- LAIV4 may be administered simultaneously with other inactivated or live vaccines. If not given simultaneously, then ≥4 weeks should pass between administration of LAIV4 and another live vaccine.
- Injectable vaccines given simultaneously should be administered at separate anatomic sites.
- Data on the immunogenicity and safety of simultaneous or sequential administration of two nonaluminum adjuvant–containing vaccines are limited.
- For newer vaccines, data informing coadministration with influenza vaccines might be limited or evolving. Providers should consult current CDC/ACIP recommendations and guidance for up-to-date information.
- VAERS is the national vaccine safety monitoring system co-managed by CDC and FDA, which serves as an early warning system to detect possible safety problems with U.S. vaccines.
- Health care providers are required to report to VAERS any adverse event listed by the vaccine manufacturer as a contraindication to further doses of vaccine and adverse events listed online [65 KB, 5 pages].
- For information on how to report to VAERS, go the VAERS website.
CDC Influenza Information (for more, call 800-232-4636)
- General CDC Influenza page
- FluView (weekly U.S. surveillance)
- Influenza Antiviral Guidance
- COVID-19 vaccination recommendations
- Vaccine Storage and Handling Toolkit
- Recommendations for Prevention and Control of Influenza in Children, 2023–2024
- IDSA Guidance for vaccination of immunocompromised hosts
- Manufacturer package inserts for U.S.-licensed vaccines
Available Influenza Vaccines, Age Indications, Dosage and Administration, and Contraindications and Precautions
Table 1: Inactivated Influenza Vaccines (IIV4s) and Recombinant Influenza Vaccine (RIV4)
| Trade name Manufacturer |
Available presentations | Approved age indications | Volume per dose by age group | ||||
|---|---|---|---|---|---|---|---|
| Quadrivalent IIVs (IIV4s)—Standard-dose—Egg-based (15 µg HA per virus component in 0.5 mL; 7.5 µg HA per virus component in 0.25 mL) | |||||||
| Afluria Quadrivalent Seqirus |
0.5 mL prefilled syringe | ≥3 yrs† | ≥3 yrs—0.5 mL† 6 through 35 mos—0.25 mL† |
||||
| 5.0 mL multi-dose vial* | ≥6 mos (needle/syringe)† 18 through 64 yrs (jet injector) |
||||||
| Fluarix Quadrivalent GlaxoSmithKline |
0.5 mL prefilled syringe | ≥6 mos | ≥6 mos—0.5 mL | ||||
| FluLaval Quadrivalent
GlaxoSmithKline |
0.5 mL prefilled syringe | ≥6 mos | ≥6 mos—0.5 mL | ||||
| Fluzone Quadrivalent Sanofi Pasteur |
|||||||
| 0.5 mL prefilled syringe | ≥6 mos§ | ≥3 yrs—0.5 mL§
6 through 35 mos—0.25 mL or 0.5 mL§ |
|||||
| 0.5 mL single-dose vial | ≥6 mos§ | ||||||
| 5.0 mL multi-dose vial* | ≥6 mos§ | ||||||
| Quadrivalent IIV (ccIIV4)—Standard-dose—Cell culture-based (15 µg HA per virus component in 0.5 mL) | |||||||
| Flucelvax Quadrivalent
Seqirus |
0.5 mL prefilled syringe | ≥6 mos | ≥6 mos—0.5 mL | ||||
| 5.0 mL multi-dose vial* | ≥6 mos | ||||||
| Quadrivalent IIV (HD-IIV4)—High dose—Egg-based (60 µg HA per virus component in 0.7 mL) | |||||||
| Fluzone High-Dose Quadrivalent Sanofi Pasteur |
0.7 mL prefilled syringe | ≥65 yrs | ≥65 yrs—0.7 mL | ||||
| Adjuvanted quadrivalent IIV4 (aIIV4)—Standard-dose with MF59 adjuvant — Egg-based (15 µg HA per virus component in 0.5 mL) | |||||||
| Fluad Quadrivalent Seqirus |
0.5 mL prefilled syringe | ≥65 yrs | ≥65 yrs—0.5 mL | ||||
| Quadrivalent RIV (RIV4) – Recombinant HA (45 µg HA per virus component in 0.5 mL) | |||||||
| Flublok Quadrivalent Sanofi Pasteur |
0.5 mL prefilled syringe | ≥18 yrs | ≥18 yrs—0.5 mL | ||||
HA= Hemagglutinin
* Contains thimerosal as a preservative agent.
† The approved dose volume for Afluria Quadrivalent is 0.25 mL for children aged 6 through 35 months and 0.5 mL for persons aged ≥3 years. However, 0.25-mL prefilled syringes are no longer available. For children aged 6 through 35 months, a 0.25-mL dose must be obtained from a multidose vial.
§ Per the package insert, Fluzone Quadrivalent is currently approved for children aged 6 through 35 months at either 0.25 mL or 0.5 mL per dose; however, 0.25-mL prefilled syringes are no longer available. If a prefilled syringe of Fluzone Quadrivalent is used for a child in this age group, the dose volume will be 0.5 mL per dose.
Administration of IIV4 and RIV4
- IIV4s and RIV4 are administered intramuscularly (IM). For adults and older children, the deltoid is the preferred site. For infants and younger children, the anterolateral thigh is the preferred site. Detailed guidance for administration sites and needle length is available in the General Best Practice Guidelines on Immunization.
- RIV4 is licensed for persons aged ≥18 years and should not be used for children and adolescents aged <18 years.
- HD-IIV4 and aIIV4 are licensed for persons aged ≥65 years and should not be used for persons aged <65 years.
Table 2: Live Attenuated Influenza Vaccine (LAIV4)
| Trade Name
Manufacturer |
Available presentations | Approved age indication | Volume per dose | |
|---|---|---|---|---|
| Quadrivalent LAIV (LAIV4) – Egg-based (contains 106.5-7.5 fluorescent focus units/0.2 mL) | ||||
| FluMist Quadrivalent
AstraZeneca |
0.2 mL prefilled single-use intranasal sprayer |
2 through 49 yrs | 0.1 mL each nostril (0.2 mLtotal) | |
Administration of LAIV4
- LAIV4 is administered intranasally using the supplied prefilled, single-use sprayer containing 0.2 mL of vaccine.
- Half of the total sprayer contents is sprayed into the first nostril while the recipient is in the upright position.
- The attached divider clip is removed and the second half of the dose administered into the other nostril.
- If the vaccine recipient sneezes immediately after administration, the dose should not be repeated.
- If nasal congestion is present that might interfere with delivery of the vaccine to the nasopharyngeal mucosa, deferral should be considered, or another age-appropriate vaccine should be administered.
Table 3: Influenza Vaccine Contraindications and Precautions
| Egg-based IIV4s | Contraindications:
Precautions:
|
|---|---|
| ccIIV4 | Contraindications:
Precautions:
|
| RIV4 | Contraindications:
Precautions:
|
| LAIV4 | Contraindications:
Precautions:
|
Table 4: Contraindications and Precautions for Persons with a History of Severe Allergic Reaction to an Influenza Vaccine
| Vaccine (of any valency) associated with previous severe allergic reaction (e.g., anaphylaxis) |
Available 2023–24 influenza vaccines | ||
|---|---|---|---|
| Egg-based IIV4s and LAIV4 | ccIIV4 | RIV4 | |
| Any egg-based IIV or LAIV | Contraindication* | Precaution† | Precaution† |
| Any ccIIV | Contraindication* | Contraindication* | Precaution† |
| Any RIV | Contraindication* | Precaution† | Contraindication* |
| Unknown influenza vaccine | Allergist consultation recommended | ||
*When a contraindication is present, a vaccine should not be administered. In addition to the contraindications based on history of severe allergic reaction to influenza vaccines noted in the Table, each individual influenza vaccine is contraindicated for persons who have had a severe allergic reaction (e.g., anaphylaxis) to any component of that vaccine. Vaccine components can be found in package inserts. Although a history of severe allergic reaction (e.g., anaphylaxis) to egg is a labeled contraindication to the use of egg-based IIV4s and LAIV4, ACIP makes an exception for allergy to egg (see Persons with Egg Allergy).
†When a precaution is present, vaccination should generally be deferred but might be indicated if the benefit of protection from the vaccine outweighs the risk for an adverse reaction. Providers can consider using the following vaccines in these instances; however, vaccination should occur in an inpatient or outpatient medical setting with supervision by a health care provider who is able to recognize and manage severe allergic reactions: 1) for persons with a history of severe allergic reaction (e.g., anaphylaxis) to any egg-based IIV or LAIV of any valency, the provider can consider administering ccIIV4 or RIV4; 2) for persons with a history of severe allergic reaction (e.g., anaphylaxis) to any ccIIV of any valency, the provider can consider administering RIV4; and 3) for persons with a history of severe allergic reaction (e.g., anaphylaxis) to any RIV of any valency, the provider can consider administering ccIIV4. Providers can also consider consulting with an allergist to help determine which vaccine component is responsible for the allergic reaction.
- Main influenza vaccine types:
- IIV= Inactivated Influenza Vaccine
- RIV= Recombinant Influenza Vaccine
- LAIV= Live Attenuated Influenza Vaccine
- Numerals after letters indicate valency (the number of influenza viruses represented):
- 4 for quadrivalent vaccines
- 3 for trivalent vaccines
- Prefixes are sometimes used to refer to specific IIVs:
- a for adjuvanted IIV (e.g., aIIV4)
- cc for cell culture-based IIV (e.g., ccIIV4)
- HD for high-dose IIV (e.g., HD-IIV4)
- SD for standard-dose IIV (e.g., SD-IIV4)
