
Trichinellosis
[Trichinella spp.]
Causal Agents
Trichinellosis (trichinosis) is caused by nematodes (roundworms) of the genus Trichinella. In addition to the classical agent T. spiralis (found worldwide in many carnivorous and omnivorous animals), several other species of Trichinella are now recognized, including T. pseudospiralis (mammals and birds worldwide), T. nativa (Arctic bears), T. nelsoni (African predators and scavengers), T. britovi (carnivores of Europe and western Asia), and T. papuae (wild and domestic pigs, Papua New Guinea and Thailand). Trichinella zimbabwensis is found in crocodiles in Africa but to date there are no known associations of this species with human disease.
Life Cycle

Trichinellosis is caused by the ingestion of undercooked meat containing encysted larvae (except for T. pseudospiralis and T. papuae, which do not encyst) of Trichinella species  . After exposure to gastric acid and pepsin, the larvae are released from the cysts
. After exposure to gastric acid and pepsin, the larvae are released from the cysts  and invade the small bowel mucosa where they develop into adult worms
and invade the small bowel mucosa where they develop into adult worms  . Females are 2.2 mm in length; males 1.2 mm. The life span in the small bowel is about four weeks. After 1 week, the females release larvae
. Females are 2.2 mm in length; males 1.2 mm. The life span in the small bowel is about four weeks. After 1 week, the females release larvae  that migrate to striated muscles where they encyst
that migrate to striated muscles where they encyst 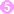 . Diagnosis is usually made based on clinical symptoms, and is confirmed by serology or identification of encysted or non-encysted larvae in biopsy or autopsy specimens.
. Diagnosis is usually made based on clinical symptoms, and is confirmed by serology or identification of encysted or non-encysted larvae in biopsy or autopsy specimens.
Geographic Distribution
Worldwide. Most common in parts of Europe and the United States.
Clinical Presentation
Light infections may be asymptomatic. Intestinal invasion can be accompanied by gastrointestinal symptoms (diarrhea, abdominal pain, vomiting). Larval migration into muscle tissues (one week after infection) can cause periorbital and facial edema, conjunctivitis, fever, myalgias, splinter hemorrhages, rashes, and peripheral eosinophilia. Occasional life-threatening manifestations include myocarditis, central nervous system involvement, and pneumonitis. Larval encystment in the muscles causes myalgia and weakness, followed by subsidence of symptoms.
Encysted larvae of Trichinella in tissue, stained with hematoxylin and eosin (H&E).







Larvae of Trichinella from bear meat.






Laboratory Diagnosis
The suspicion of trichinellosis (trichinosis), based on history, clinical symptoms, and eosinophilia, can be confirmed by specific diagnostic tests, including antibody detection, muscle biopsy, and microscopy.
Antibody Detection
Immunodiagnostic tests currently available in the U.S. include enzyme immunoassays (EIA) that detect Trichinella-specific antibodies. EIAs utilize antigen preparations that may be crude extracts prepared from homogenates of T. spiralis muscle larvae or excretory-secretory (ES) products produced by cultured larvae. The TSL-1 group of larval secretory antigens are conserved in all species/isolates of Trichinella and thus can be used to detect infection in animals or people infected with any of the types of Trichinella currently recognized. Positive reactions are detectable at some time during infection in serum samples of 80% to 100% of patients with clinically symptomatic trichinellosis (trichinosis). Antibody levels are often not detectable until 3 to 5 weeks post-infection, well after the onset of acute-stage illness. Antibody development is also affected by the infecting dose of larvae: the higher the infecting dose, the faster the patient’s antibody response will develop. Multiple serum specimens should be drawn several weeks apart to demonstrate seroconversion in patients whose initial specimen was negative. IgG, IgM, and IgE antibodies are detectable in many patients; however, tests based on IgG antibodies are most sensitive. Antibody levels peak in the second or third month post-infection and then decline slowly for several years. In our experience at CDC, EIAs with ES antigens detect antibodies earlier than bentonite flocculation (BF) in 25% of serum specimens from patients with acute infection, but EIAs also remain positive for longer periods after infection than the BF, and are reactive in a larger proportion of persons with no clinical evidence of trichinellosis.
Reference:
Murrell KD, Bruschi F. Clinical trichinellosis. Prog Clin Parasitol 1994;4:117-150.
Treatment Information
Treatment information for trichinellosis can be found at:
Clinical Care of Trichinellosis
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.

